Abstract
Aims
To quantify greyzone fibrosis (GZF) in patients after acute myocardial infarction (MI) and to evaluate its correlation with MI-free survival and improvements in left ventricular ejection fraction (LVEF) compared with the established risk factors high-sensitivity cardiac troponin T (hs-cTnT) and Late Gadolinium Enhancement (LGE).
Methods and results
The study involved 176 patients who experienced acute MI and underwent cardiac magnetic resonance (CMR) prior to hospital discharge, followed by a second CMR on average six months later. LGE was quantified in both examinations, a separate analysis of the GZF was conducted only in the follow-up CMR after resolution of the initial infarct edema. LVEF was measured in both CMR. hs-cTnT levels were assessed at hospital admission, as well as 8, 16, 24, 48 and 72 h after coronary intervention. Telephone follow-ups were conducted annually for up to 8 years. LGE measurements showed better correlation with MI-free survival (Harrell’s C of 0.711 of LGE mass) compared to GZF (0.579 of GZF mass). Additionally, hs-cTnT outperformed GZF (Harrell’s C of 0.645). As an univariable predictor for MI-free survival, only hs-cTnT reached significance (p < 0.05). With regard to improvements in ejection fraction, both hs-cTnT and LGE measurements showed acceptable correlation with improvement in ejection fraction (p < 0.05), while GZF measurements showed no correlation (p > 0.5).
Conclusions
In CMR, the assessment of GZF demonstrated inferior p correlation compared to hs-cTnT and LGE in patients after acute MI with respect to the endpoint of MI-free survival. Furthermore, GZF showed no correlation with the improvement of LVEF.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, risk stratification for patients with myocardial infarction (MI) or ischemic heart disease is mostly based on left ventricular ejection fraction (LVEF), e.g., the recommendation for an implantable cardioverter-defibrillator (ICD) [1, 2].
However, other risk factors like high levels of high-sensitivity cardiac troponin T (hs-cTnT), presence of myocardial fibrosis (Late Gadolinium Enhancement, LGE), hypertension or diabetes for increased mortality after MI have also been described [3,4,5]. So-called greyzone fibrosis (GZF) can be detected by cardiac magnetic resonance (CMR) and consists of myocardial fibrosis and viable myocardium [6]. It is described as a risk factor for life-threatening arrhythmias, such as ventricular fibrillation in patients with coronary artery disease [3, 6].
So far, data about the correlation between the amount of GZF occurring after MI and long-term clinical parameters are scarce. Thus, the purpose of this study was to quantify GZF and to evaluate its correlation with survival and improvements in LVEF in a well-characterized cohort with an index event. The study furthermore aimed at comparing these correlations with those of the established risk factors hs-cTnT and Late Gadolinium Enhancement.
Methods
This retrospective study included patients who experienced myocardial infarction type I (ST-segment elevation and non-ST-segment elevation myocardial infarction [7]) from September 2014 to November 2019. These patients underwent CMR prior to hospital discharge, followed by a second CMR on average six months later. Patients with poor image quality at CMR were excluded from the analyses. All clinical data and follow-up (FU) information were sourced from our institutional database.
High-sensitivity cardiac troponin T (hs-cTnT) was measured (cobas pro, Roche Germany Holding GmbH, cutoff value < 0.014 ng/ml) at admission, 8, 16, 24, 48 and 72 h after PCI and patients were monitored using a standardized follow-up protocol, including annual phone calls for up to 8 years to determine adverse events such as death, myocardial infarction, stroke, bleeding, and hospitalizations. All patients gave their written informed consent for the anonymized use of clinical, procedural and follow-up data at the time of the intervention. This study was approved by the institutional review board and complied with the Declaration of Helsinki.
Cardiac magnetic resonance imaging
All CMR-examinations were performed on a 3.0 Tesla scanner (Siemens Magnetom Skyra, Siemens Healthineers, Forchheim, Germany) with patients placed in supine position and using a cardiac coil. Images were acquired at end-expiratory breath hold. A bolus of contrast agent was applied (0.2 ml/kg bodyweight, Magnevist®, Bayer Pharma, Berlin, Germany). 5 min after the bolus, retrospectively gated contrast-enhanced steady-state free precision (SSFP) cine images in short-axis (SAX) stack covering the left ventricle from the base to apex, 2-, 3- and 4-chamber view were acquired. Image parameters for SSFP cines were: TE 1.4 ms; TR 2.9 ms; flip angle 60°; image resolution 1.5 × 1.5 × 8 mm; slice gap 0 mm. No parallel imaging was performed to maximize the signal-to-noise ratio (SNR). 15 min after contrast injection, late gadolinium enhancement (LGE) images were acquired in the same planes as cine images with a phase-sensitive inversion-recovery sequence (TE 3.3 ms, TR 7.0 ms, TI 250–500 ms to null the myocardium, 8 mm slice, no gap, matrix 256 × 192).
Image analysis
Image analysis was performed with dedicated post-processing workstations (syngo.via, Siemens Healthineers AG, Forchheim, Germany; CVI42, Circle Cardiovascular Imaging Inc, Calgary, AB, Canada) by two experienced readers (P.B. and P.R. with > 3 years of experience in CMR and both certified with the highest degree in CMR of the German Cardiac Society) independently.
Volume measurements, LVEF, stroke volume, cardiac index and myocardial mass were semi-automatically assessed using the SSFP-cine images. For all analyses, the endocardial and epicardial borders of the left ventricle were manually traced in all short-axis slices in end-diastole and end-systole. The papillary muscles were excluded from the myocardium. Improvement of LVEF was defined as the delta between LVEF in CMR 1 and in CMR 2. As a parameter for left ventricular remodeling we defined the delta between left ventricular end-diastolic volume (LVEDV) in CMR 1 and in CMR 2.
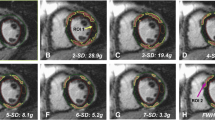
In order to quantify the myocardial edema in the contrast-enhanced SSFP-cine images [8] of first CMR, a semiautomatic delineation using signal-intensity (SI) thresholds of the hyperintense, edematous region (SI > 2 SD exceeding the mean SI of remote myocardium) was performed in all short-axis slices (in systole). The infarct area was semi-automatically assessed in the short-axis LGE images in both CMR (SI > 5 SD exceeding the mean SI of remote myocardium) (Fig. 1). A hypointense signal within the area of LGE representing microvascular obstruction, if present, was included in the analysis. All automatically assessed areas were visually controlled and adjusted if necessary.
For GZF analysis, the mean SI of remote myocardium was adopted to 3 SD SI and the area of enhanced myocardium was semi-automatically assessed. GZF was calculated as follows: 3 SD SI—LGE.
Statistical analysis
All statistical analyses were performed using Stata (StataCorp LCC, Texas, USA, version 18).
Categorical variables are expressed as frequencies and percentages, continuous variables as mean and standard deviation (SD) or median with interquartile range (IQR). For MI-free survival, univariable and bivariable Cox regression models were conducted, and Harrell’s C and Royston and Sauerbrei’s D were calculated as discrimination measures and compared across different potential predictors. For improvements in ejection fraction, univariable and bivariable linear regression models were conducted and the coefficients of determination R2 were calculated and compared. A value of p < 0.05 was considered as statistically significant. Observer agreement was assessed using the intraclass correlation coefficient, with values above 0.90 indicating excellent reliability.
Results
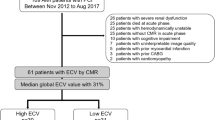
176 patients (18.2% female, 63.3 ± 11.4 years) with acute myocardial infarction (114 with ST-segment elevation myocardial infarction (STEMI), 62 with non-ST-segment elevation myocardial infarction (NSTEMI)) were included in this study. Mean time between MI and CMR 1 was 3 ± 1 days and between MI and CMR 2 191 ± 65 days. Median time between CMR 1 and CMR 2 was 186 days [interquartile range, IQR, 181; 194].
Median hs-cTnT-level at admission was 0.240 ng/ml [0.072; 0.665] and median peak hs-cTnT-level was 2.835 ng/ml [1.043; 5.212]. CMR analyses showed a mean LVEF of 49.8 ± 9.7% at admission (= CMR 1) and of 55.1 ± 9.7% at follow-up (= CMR 2). Interobserver reliability was high for CMR-measurement of LVEF [intraclass correlation coefficient 0.973 (95% confidence interval [CI]: 0.912–0.991)].
Mean LGE mass was 14.21 ± 11.98 g, mean GZF 4.77 ± 2.89 g. Patients with STEMI had significant higher levels of hs-cTnT at any timepoint as well as higher amounts of LGE (p < 0.001 each) than patients with NSTEMI. There were no significant differences between patients with STEMI and NSTEMI regarding the amount of myocardial edema (p = 0.125) and GZF (p = 0.253).
All baseline characteristics including the treatment of the acute myocardial infarction are summarized in Table 1, and hs-cTnT-levels and CMR analyses are summarized in Table 2.
Follow-up
No patient died or suffered a second myocardial infarction in the period between CMR 1 and CMR 2. During the median follow-up period of 1132 days [1093; 1331], six patients died (3.4%, 3 from neurological diseases, 1 from pneumonia, 1 from heart failure and 1 from myocardial infarction). Seven patients suffered a second myocardial infarction (4.0%, Fig. 2). During follow-up, 158 patients (89.8%) were hospitalized for a planned coronary angiography. All follow-up data are summarized in Table 3.
Regression analyses and analyses for predictive power showed that LGE measurements were associated with better correlation (Harrell’s C of 0.711 of LGE mass) compared to GZF (0.579 GZF) regarding MI-free survival. hs-cTnT at admission also performed better (Harrell’s C of 0.645) than GZF and peak hs-cTnT or hs-cTnT 8, 16, 24, 48 and 72 h after PCI. As an univariable predictor for MI-free survival, only hs-cTnT at admission reached significance (p = 0.002) (Table 4, supplementary tables 1, 2 and 3).
With regard to improvements in ejection fraction, hs-cTnT, LGE and MVO measurements as well as the presence of STEMI showed acceptable correlation with improvement in ejection fraction (p < 0.05), but GZF measurements showed no correlation (p > 0.05) (Table 5). Regarding left ventricular remodeling, hs-cTnT, GZF, LGE and MVO showed acceptable correlation with improvement in LVEDV (p < 0.05), but GZF showed worse R squared values than LGE and hs-cTnT (GZF < 0.2, hs-cTnT and LGE > 0.2) (supplementary Table 4).
Discussion
To the best of our knowledge, our study is the first to evaluate an association between GZF and the parameters MI-free survival and improvement of the LVEF in the long-term follow-up after acute myocardial infarction. In our analyses, hs-cTnT and LGE measurements revealed the best correlation with these endpoints. GZF failed as suitable predictor.
Ventricular arrhythmias and the development of ischemic heart disease are the most common complications of a MI and represent a relevant health burden due to hospitalization of the patients and costs for drug therapy or devices such as ICDs [9, 10]. Thus, individualized therapy is required, starting with the coronary intervention in acute MI and continuing through to follow-up care [10]. As research regarding MI is evolving, e.g., a new MI classification based on the different mechanism of tissues injury in MI [11], an individualized risk stratification after MI including biomarkers as well as imaging methods is needed. Currently, biomarkers such as hs-cTnT and LVEF are routinely used to assess the risk for post-MI events [2]. Previous studies demonstrated a strong correlation between troponin levels and a lower LVEF in the follow-up [12]. These results were reproducible in our study.
However, the accuracy of biomarkers and echocardiography are often limited by patient individual factors such as renal insufficiency (influencing troponin levels) or poor image quality due to adiposity.
CMR enables a reliable assessment of cardiac function, cardiac structure (e.g., scar development or microvascular obstruction), as well as the detection of left ventricular thrombi and has shown promising results for estimating the individual risk of complications after MI [10, 13]. Yet, CMR methods post-MI vary widely and CMR is not routinely recommended after MI [10]. Given the high costs and the low availability, a targeted use in patients who will benefit most is desirable. LVEF assessed by CMR, assessment of intramural hemorrhage and LGE quantification are known as most valuable analyses after MI with good correlations with all-cause mortality or heart failure-driven hospitalization and with biomarkers [14,15,16,17,18]. This is in line with our findings that LGE had a good correlation with MI-free survival and improvement in LVEF. In our analyses, the presence of STEMI (in contrast to NSTEMI) showed a correlation with the improvement in LVEF. This is most likely attributed to a greater infarct size and area at risk in STEMI patients, which has been described previously [19].
The assessment of LVEF by CMR has been reported to provide excellent reliability [20]. We were able to report a similar interobserver variability for our LVEF measurements indicating a high consistency across all patients.
In contrast, the assessment of myocardial edema and especially the GZF have been discussed controversially [15]. One reason for this might be the inconsistency in methodology based on different signal intensities attributed to GZF [6, 15].
Small studies have shown an association between GZF and ventricular arrhythmias in patients with previous MI and LVEF < 35% [21,22,23,24].
A large previous study of 979 patients with chronic coronary syndrome showed an association of the myocardial fibrosis and GZF with ventricular arrhythmias and sudden cardiac death—independent of LVEF [6]. Another study described GZF as none superior with regard to diagnostic accuracy over LGE [22].
In contrast to these data, GZF showed neither correlation with improvement of the LVEF nor with MI-free survival in our study. Hence, our data make GZF quantification seem dispensable after MI.
Limitations
Several limitations of our study must be considered. First, we report on a retrospective study. The limited size of the study cohort and the small amount of second MI during follow-up restrict the power of the analyses. Thus, we cannot exclude an existing minor association. However, as other parameters showed a good prediction of our endpoints, we assume that potential minor associations can be neglected. Furthermore, we were not able to include death as an endpoint due to the low events. Since we report a retrospective study from 2014 to 2019, we cannot analyze T1 and T2 mapping data, as these images were not acquired at our institution during that period. Furthermore, we did not analyze intramural hemorrhage. Mean time between CMR 1 and CMR 2 varied to up to 1 year due to logistical reasons, representing routine clinical processes. Nevertheless, since our findings were consistent, we assume that there was no significant impact on our main results. The high number of re-hospitalizations can be attributed to these patients either undergoing staged coronary intervention for residual stenosis or undergoing follow-up coronary angiography after intervention for acute myocardial infarction, both of which were standard practices at our institution during this period.
Conclusion
The greyzone fibrosis analysis does not add predictive value of CMR in patients after MI, as it shows worse correlation compared to hs-cTnT and LGE regarding the MI-free survival. Furthermore, GZF is not suitable as predictor of improvement of LVEF.
Data Availability
The data are not publicly available due to containing information that could compromise the privacy of the research participants.
References
Al-Khatib SM, Stevenson WG, Ackerman MJ et al (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Circulation 138:e210–e271. https://doi.org/10.1161/CIR.0000000000000548
Zeppenfeld K, Tfelt-Hansen J, de Riva M et al (2022) 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 43:3997–4126. https://doi.org/10.1093/eurheartj/ehac262
Zegard A, Okafor O, de Bono J et al (2021) Myocardial fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol 77:29–41. https://doi.org/10.1016/j.jacc.2020.10.046
Chunawala ZS, Caughey MC, Bhatt DL et al (2023) Mortality in patients hospitalized with acute myocardial infarction without standard modifiable risk factors: the ARIC Study Community Surveillance. J Am Heart Assoc 12:e027851. https://doi.org/10.1161/JAHA.122.027851
Kaldal A, Tonstad S, Jortveit J (2023) Association of troponin T measurements with long-term outcomes in patients with coronary artery disease participating in a secondary prevention trial. BMC Cardiovasc Disord 23:210. https://doi.org/10.1186/s12872-023-03249-0
Zegard A, Okafor O, de Bono J et al (2022) Greyzone myocardial fibrosis and ventricular arrhythmias in patients with a left ventricular ejection fraction >35. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol 24:31–39. https://doi.org/10.1093/europace/euab167
Thygesen K, Alpert JS, Jaffe AS et al (2019) Fourth universal definition of myocardial infarction (2018). Eur Heart J 40:237–269. https://doi.org/10.1093/eurheartj/ehy462
Ubachs JFA, Sörensson P, Engblom H et al (2012) Myocardium at risk by magnetic resonance imaging: head-to-head comparison of T2-weighted imaging and contrast-enhanced steady-state free precession. Eur Heart J Cardiovasc Imaging 13:1008–1015. https://doi.org/10.1093/ehjci/jes091
Winkler C, Funk M, Schindler DM et al (2013) Arrhythmias in patients with acute coronary syndrome in the first 24 hours of hospitalization. Heart Lung J Crit Care 42:422–427. https://doi.org/10.1016/j.hrtlng.2013.07.010
Byrne RA, Rossello X, Coughlan JJ et al (2023) 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 44:3720–3826. https://doi.org/10.1093/eurheartj/ehad191
Kumar A, Connelly K, Vora K et al (2024) The Canadian Cardiovascular Society classification of acute atherothrombotic myocardial infarction based on stages of tissue injury severity: an expert consensus statement. Can J Cardiol 40:1–14. https://doi.org/10.1016/j.cjca.2023.09.020
Hallén J, Jensen JK, Fagerland MW et al (2010) Cardiac troponin I for the prediction of functional recovery and left ventricular remodelling following primary percutaneous coronary intervention for ST-elevation myocardial infarction. Heart Br Card Soc 96:1892–1897. https://doi.org/10.1136/hrt.2009.190819
Disertori M, Rigoni M, Pace N et al (2016) Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging 9:1046–1055. https://doi.org/10.1016/j.jcmg.2016.01.033
Stone GW, Selker HP, Thiele H et al (2016) Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol 67:1674–1683. https://doi.org/10.1016/j.jacc.2016.01.069
Ibanez B, Aletras AH, Arai AE et al (2019) Cardiac MRI Endpoints in myocardial infarction experimental and clinical trials: JACC scientific expert panel. J Am Coll Cardiol 74:238–256. https://doi.org/10.1016/j.jacc.2019.05.024
El Aidi H, Adams A, Moons KGM et al (2014) Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol 63:1031–1045. https://doi.org/10.1016/j.jacc.2013.11.048
Schneider JN, Neumann JT, Bohnen S et al (2021) Association of late gadolinium enhancement with biomarkers in patients with myocardial infarction. Coron Artery Dis 32:730. https://doi.org/10.1097/MCA.0000000000001034
Lechner I, Reindl M, Stiermaier T et al (2024) Clinical outcomes associated with various microvascular injury patterns identified by CMR after STEMI. J Am Coll Cardiol 83:2052–2062. https://doi.org/10.1016/j.jacc.2024.03.408
Figueras J, Otaegui I, Marti G et al (2018) Area at risk and collateral circulation in a first acute myocardial infarction with occluded culprit artery. STEMI vs non-STEMI patients. Int J Cardiol 259:14–19. https://doi.org/10.1016/j.ijcard.2018.01.047
Lapinskas T, Hireche-Chikaoui H, Zieschang V et al (2019) Effect of comprehensive initial training on the variability of left ventricular measures using fast-SENC cardiac magnetic resonance imaging. Sci Rep 9:12223. https://doi.org/10.1038/s41598-019-48685-1
Acosta J, Fernández-Armenta J, Borràs R et al (2018) Scar characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT study. JACC Cardiovasc Imaging 11:561–572. https://doi.org/10.1016/j.jcmg.2017.04.021
de Haan S, Meijers TA, Knaapen P et al (2011) Scar size and characteristics assessed by CMR predict ventricular arrhythmias in ischaemic cardiomyopathy: comparison of previously validated models. Heart 97:1951–1956. https://doi.org/10.1136/heartjnl-2011-300060
Demirel F, Adiyaman A, Timmer JR et al (2014) Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int J Cardiol 177:392–399. https://doi.org/10.1016/j.ijcard.2014.08.132
Zeidan-Shwiri T, Yang Y, Lashevsky I et al (2015) Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm 12:802–808. https://doi.org/10.1016/j.hrthm.2015.01.007
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart; formal analysis: Ramona Schmitt, Clara Staats, Klaus Kaier, Christoph Ahlgrim, Manuel Hein, Johannes Brado, Philipp Steinhoff, Hannah Billig, Martin Soschynski, Tobias Krauss, Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart; investigation: Ramona Schmitt, Clara Staats, Klaus Kaier, Manuel Hein, Philipp Steinhoff, Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart; methodology: Manuel Hein, Philipp Ruile and Philipp Breitbart; resources: Christopher L. Schlett, Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart; software: Klaus Kaier, Christopher L. Schlett; supervision: Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart; validation: Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart; visualization: Ramona Schmitt, Clara Staats and Klaus Kaier; writing—original draft: Ramona Schmitt and Philipp Breitbart; writing—review and editing: Ramona Schmitt, Clara Staats, Klaus Kaier, Christoph Ahlgrim, Manuel Hein, Johannes Brado, Philipp Steinhoff, Hannah Billig, Martin Soschynski, Tobias Krauss, Christopher L. Schlett, Dirk Westermann, Franz-Josef Neumann, Philipp Ruile and Philipp Breitbart. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Albert-Ludwigs-University Freiburg (protocol code 228/14, date of approval: 06 June 2014).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmitt, R., Staats, C., Kaier, K. et al. Correlation of greyzone fibrosis compared to troponin T and late gadolinium enhancement with survival and ejection fraction in patients after acute myocardial infarction. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02536-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02536-w