Abstract
Background
Aging as a major non-modifiable cardiac risk factor challenges future cardiovascular medicine and economic demands, which requires further assessments addressing physiological age-associated cardiac changes.
Objectives
Using cardiovascular magnetic resonance (CMR), this study aims to characterize sex-specific ventricular adaptations during healthy aging.
Methods
The population included healthy volunteers who underwent CMR at 1.5 or 3 Tesla scanners applying cine-imaging with a short-axis coverage of the left (LV) and right (RV) ventricle. The cohort was divided by sex (female and male) and age (subgroups in years): 1 (19–29), 2 (30–39), 3 (40–49), and 4 (≥50). Cardiac adaptations were quantitatively assessed by CMR indices.
Results
After the exclusion of missing or poor-quality CMR datasets or diagnosed disease, 140 of 203 volunteers were part of the final analysis. Women generally had smaller ventricular dimensions and LV mass, but higher biventricular systolic function. There was a significant age-associated decrease in ventricular dimensions as well as a significant increase in LV mass-to-volume ratio (LV-MVR, concentricity) in both sexes (LV-MVR in g/ml: age group 1 vs. 4: females 0.50 vs. 0.57, p=0.016, males 0.56 vs. 0.67, p=0.024). LV stroke volume index decreased significantly with age in both sexes, but stronger for men than for women (in ml/m2: age group 1 vs. 4: females 51.76 vs. 41.94, p<0.001, males 55.31 vs. 40.78, p<0.001). Ventricular proportions (RV-to-LV-volume ratio) were constant between the age groups in both sexes.
Conclusions
In both sexes, healthy aging was associated with an increase in concentricity and a decline in ventricular dimensions. Furthermore, relevant age-related sex differences in systolic LV performance were observed.
Graphical Abstract
↓, decrease; ↑, increase; ±, maintaining. Abbreviations: CMR, cardiovascular magnetic resonance; EDV, end-diastolic volume; EF, ejection fraction; LV, left ventricle; MVR, mass-to-volume ratio; RV, right ventricle; SVI, stroke volume index; T, Tesla; VR, volume ratio.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of cardiovascular diseases increases with rising age [1, 2]. Global demographic change will likely impact not just patient’s health but also lead to an increasing burden on healthcare resources [2]. Therefore, research into the physiological role of non-modifiable risk factors such as age and sex becomes increasingly important. It is known that left ventricular (LV) remodeling occurs in patients with underlying cardiac diseases such as myocardial infarction [3]. Data also suggest that rising age is associated with LV remodeling [4, 5] including an increased occurrence of LV fibrosis [6], stiffness [7], and wall thickening [8, 9], turning age into one of the strongest cardiovascular risk factors for the development of cardiovascular diseases and particularly heart failure (HF). Sex-specific differences in the general and age-associated cardiac morphology are also reported in literature: Female hearts tend to be more affected by concentric than eccentric remodeling [10, 11], by a greater increase in LV wall thickening [7, 11, 12] and diastolic dysfunction [11, 13, 14] compared to male hearts, which is also reflected in the clinical phenotype of HF. Women display a higher prevalence of HF with preserved ejection fraction, whereas men are more likely to develop HF with reduced ejection fraction [15].
Nevertheless, data regarding the age-related progression of LV systolic performance and LV mass (LVM) are discordant and also vary in their methodology [8, 9, 16, 17]. Potential age-related cardiac changes should be investigated across all life stages and with equal distribution of sex, particularly as women are underrepresented in cardiovascular research [18] and appear to show a higher in-hospital mortality rate (as assessed in a cohort with myocardial infarction) [19]. Further investigation of the impact of sex and age on cardiac geometry and function will improve the understanding of physiological ventricular adjustments over the adult lifetime and could provide possible explanations for sex differences in cardiac morbidity and mortality.
Since cardiovascular magnetic resonance (CMR) has been established as the gold standard for the quantification of cardiac chambers as well as for myocardial tissue characterization [20], it is highly qualified to evaluate cardiac changes during healthy aging. Quantitative CMR parameters provide the basis for ventricular and atrial assessment and allow the differentiation of pathological from physiological conditions. Indexed and related performance parameters, such as the LV mass-to-volume ratio (LV-MVR) as a predictor of concentricity, the LV stroke volume index (LV-SVI) as a determinant of cardiac output, and the ratio of right ventricular (RV) to LV end-diastolic volumes (RV/LV-VR) to evaluate the proportion of ventricular dimensions, can provide adequate diagnostic as well as prognostic [21,22,23] information.
This study aims to characterize physiological age-dependent cardiac adaptations in ventricular geometry and function between healthy women and men by using quantitative volumetric and functional indices in CMR.
Methods
Study design
This research was designed as a retrospective analysis of CMR image data in a cohort of consecutively included healthy volunteers. All data were collected in previous prospective clinical trials between 2011 and 2023. These studies were approved by the ethics committee of the Charité University Medicine Berlin, Germany, and were subject to the ethical standards of the institution and the Declaration of Helsinki.
Study population
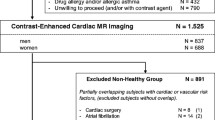
The study cohort included adult Caucasians who were enrolled as healthy controls in previous prospective studies. Participants who displayed or developed cardiovascular symptoms or diseases were consequently excluded from the cohort using a standard operating procedure (supplementary methods 1). Exclusion criteria were further extended to conditions beyond the cardiovascular system and post-analysis detected abnormalities. Thus, the final analysis covered individuals who exhibited entirely healthy conditions. Detailed information regarding the sample enrollment and the specific conditions leading to an exclusion are provided in supplementary methods 1. The total cohort was divided into different age groups (in years: 1, 19–29; 2, 30–39; 3, 40–49; 4, ≥50; adjusted after Kawel-Boehm et al. [24]) and divided by biological sex (female, male).
Image acquisition
CMR examinations were performed at 1.5 Tesla (Avanto and AvantoFit, Siemens Healthineers, Erlangen, Germany) and 3 Tesla scanners (SkyraFit and Verio, Siemens Healthineers, Erlangen, Germany). Following general anatomical overviews and localizers, the CMR protocol included ECG-gated cine imaging with a balanced steady-state free precession (bSSFP) sequence in a short-axis stack covering the entire LV and RV. Technical CMR sequence details are given in supplementary methods 2.
Image analysis
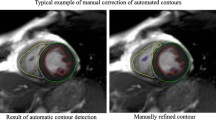
The CMR image analysis was performed using the dedicated post-processing software CVI42 (Circle CVI, version 5.13.7, Calgary, Canada). The evaluation proceeded in two phases: First, pre-existing manually generated or missing LV and RV contours from the initial studies were reworked and updated by an experienced CMR reader following a standard approach according to current scientific recommendations [20]. In a second supervision, all images were checked by two qualified CMR experts.
For short-axis biventricular assessment, endo- and epicardial borders were contoured in end-diastolic and end-systolic phases. Basal slices were counted as part of the LV blood volume if they were surrounded by at least 50% of the myocardium. LV papillary muscles were contoured in end-diastolic and end-systolic phases and counted as part of LVM.
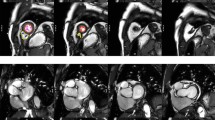
A general visual assessment of image quality was integrated into the image analysis. Referring to the image quality criteria by Klinke et al. [25], scans that displayed major artifacts (e.g. image blurring, mis-triggering, or wrap-around artifacts) were excluded from the cohort. Figure 1 illustrates a full short-axis stack contouring in the LV and RV end-diastolic phase.
Exemplary illustration of a complete cine short-axis stack contouring in LV and RV end-diastolic phases. Yellow contour: RV endocardial contour, red contour: LV endocardial contour, green contour: LV epicardial contour, purple contour: LV papillary muscle contour. Abbreviations: LV, left ventricle; RV, right ventricle
Quantitative CMR performance parameters
This study assessed ventricular adaptations during aging with a focus on indexed and related CMR parameters such as the LV-SVI, the LV-MVR, and the RV/LV-VR. LV-MVR was determined by the division of LVM and LV end-diastolic volume and RV/LV-VR by the division of RV end-diastolic volume and LV end-diastolic volume. Other parameters were indexed to both height (H) (m) and body surface area (BSA) (m2).
Statistical analysis
The statistical calculations were carried out in the software SPSS (IBM, version 28.0.0, Armonk, NY, USA). Continuous parameters were represented as mean ± standard deviation (SD), categorical variables as total, and percent (%). Normal distributions were assessed with the Shapiro-Wilk test. For the age-independent sex comparison, pairwise tests were performed using the Mann-Whitney U test for non-parametric variables and an independent sample t-test for parametric variables. The overall age group comparison was carried out by the Kruskal-Wallis test for non-parametric and by ANOVA for parametric parameters. For cases in which significance was detected, an adjusted pairwise post-hoc test followed the assessment. Categorial variables were compared using the chi-square test. Age-correlation analysis was carried out by Spearman’s rank correlation coefficient. A p-value ≤0.05 was defined as statistically significant.
Intra- and interobserver variability
For analyzing intra- and interobserver variability, the in-house developed software tool “Lazy Luna” was used, which enables automatic comparison of CMR segmentations between different readers [26, 27]. Mean deviation ± SD and Bland-Altman plots were calculated based on a subgroup of ten randomly selected subjects for biventricular volumetric and functional parameters.
Results
Baseline characteristics
A total of 203 volunteers were screened for this study. Before performing the image analysis, N=56 had to be excluded due to cardiovascular symptoms, diseases, conditions affecting other body systems, or missing CMR image data. After image analysis, another N=7 subjects were excluded due to diseases detected during post-analysis or artifacts leading to the inability of analysis. The final cohort covered N=140 healthy volunteers (Supplementary methods 1).
The cohort presented the following baseline characteristics (Table 1): Age ranged from 19 to 77 years with a cohort’s middle age ± SD of 37.73 ± 13.71 years. Females: N=77 (55%), mean age in years ± SD 39.60 ± 14.31; female age groups (in years ± SD): 1, N=20, 24.55 ± 2.96; 2, N=25, 33.80 ± 3.12; 3, N=13, 43.54 ± 3.55; 4, N=19, 60.37 ± 8.32. Males: N=63 (45%), mean age in years ± SD 35.44 ± 12.68; male age groups (in years ± SD): 1, N=25, 24.68 ± 2.87; 2, N=21, 33.57 ± 2.68; 3, N=7, 45.71 ± 3.55; 4, N=10, 59.10 ± 5.20. Height, weight, body mass index (BMI), and BSA were significantly higher in males (Table 1). Baseline characteristics of the sex-specific age groups are provided in Table 2.
Age-independent sex-specific differences in cardiac geometry and function
Males had significantly higher LV and RV volumes overall compared to females. These differences remained after indexing to BSA or H (Table 3).
Accordingly, LV-SVI (indexed to BSA/H) was significantly higher in males, although LV function as measured by ejection fraction was higher in females (Table 3).
Likewise, LVM was significantly higher in males compared to females. The same was observed after indexing to BSA or H (Table 3). For LV-MVR, males were found to have significantly higher ratios compared to females (Table 3). Furthermore, RV/LV-VR differed significantly between both sexes, but only with a slightly higher mean for males (Table 3). The overall mean for RV/LV-VR was greater than 1.0, indicating larger dimensions of the RV in relation to the LV.
Age-associated sex-specific adaptations in cardiac geometry and function
For both sexes, LV and RV volumes and their indexed parameters decreased significantly with increasing age (Tables 4, 5; Figs. 2, 3). The same was evident for LV-SVI (indexed to BSA/H): Younger individuals of both sexes were found to have significantly higher stroke volume indices than older volunteers (Tables 4, 5; Fig. 2). The analysis revealed significant negative age-related correlations for LV-SVI (indexed to BSA/H) for both sexes (Spearman’s rank correlation coefficient: LV-SVI/BSA: females −0.488, p<0.001; males −0.566, p<0.001; LV-SVI/H: females −0.409, p<0.001; males −0.375, p=0.002), confirming the decrease of LV-SVI with rising age (Fig. 4, Supplementary results 1). The decreasing LV stroke volume (index) can be explained by a greater age-related decline of LV end-diastolic volume in relation to LV end-systolic volume (Supplementary results 1). The age-correlated LV-SVI/BSA trend curves of females and males crossed at the age of 47.59 years (Fig. 4). Younger males initially presented higher LV-SVI/BSA, but lower mean values than females in advanced life stages (age groups 3 and 4). Therefore, the age-related decline of LV-SVI/BSA was stronger in males (Tables 4, 5; Fig. 4). LV-SVI/H was also found to decrease faster for men than for women with rising age, although the trend curves did not cross (Supplementary results 1).
Boxplot comparison of left ventricular CMR indices in female (left) and male (right) age groups. Boxplots represent the median (solid inside the box), interquartile range (box), and 1.5*interquartile range (whiskers). Every value below or above 1.5*interquartile range is marked as an outlier. Age groups in years: 1, 19–29 (orange); 2, 30–39 (yellow); 3, 40–49 (gray); 4, ≥50 (beige). Abbreviations: BSA, body surface area; CMR, cardiovascular magnetic resonance; EDV, end-diastolic volume; I, index; LV, left ventricle; SV, stroke volume
Boxplot comparison of right ventricular CMR indices in female (left) and male (right) age groups. Boxplots represent the median (solid inside the box), interquartile range (box), and 1.5*interquartile range (whiskers). Every value below or above 1.5*interquartile range is marked as an outlier. Age groups in years: 1, 19–29 (orange); 2, 30–39 (yellow); 3, 40–49 (gray); 4, ≥50 (beige). Abbreviations: BSA, body surface area; CMR, cardiovascular magnetic resonance, EDV, end-diastolic volume; I, index; LV, left ventricle; RV, right ventricle
RV/LV-VR was constant across all age groups in females and males, with young volunteers (age group 1) presenting almost similar RV/LV-VR parameters compared to volunteers in age group 4 (Tables 4, 5; Fig. 3). No significant correlation of RV/LV-VR with age for both sexes was found (Fig. 4).
LV-MVR increased significantly with rising age for both sexes (Tables 4, 5; Fig. 2), which was also indicated through a significant positive age-related correlation for LV-MVR (Spearman’s rank correlation coefficient: females +0.336, p=0.003, males +0.369, p=0.003, Fig. 4).
In males, LVM (and LVM indexed to BSA/H) did not differ significantly between the age groups but a trend towards an age-associated decline in LVM could be seen (Table 5; Fig. 2). Among females, LVM (and LVM indexed to BSA/H) decreased significantly between youngest (age group 1) and middle-aged groups (LVM and LVM-I/H age group 2, LVM-I/BSA age groups 2 and 3) but insignificantly between youngest (age group 1) and oldest (age group 4) subgroup (Table 4, Fig. 2).
Intra- and interobserver-variability
Bland-Altmann Plots for intra- and interobserver analysis are displayed in Figure 5. Mean deviations ± SD are provided in the supplementary results 2.
Results of intra (orange)- and interobserver (blue) analysis. The middle line represents the mean deviation, the upper line mean deviation + 1.96*SD, and the lower line mean deviation −1.96*SD. Abbreviations: LV-EDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LV-SV, left ventricular stroke volume; RV-EDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RV-SV, right ventricular stroke volume; SD, standard deviation
Discussion
This study characterized adaptations in cardiac morphology and function during healthy aging in women and men using quantitative CMR indices and found that age was associated with several ventricular changes in both sexes even in younger life decades. First, there was evidence of equal ventricular remodeling in women and men with increasing age, as represented by an increase in concentricity (LV mass-to-volume ratio). Second, LV stroke volume index as a determinant of cardiac output decreased with rising age in both sexes but declined stronger for men than for women. Third, in both sexes, chamber volumes decreased with age with a similar decline in RV and LV so that the volume ratio between both chambers remained constant over the adult lifetime.
Overall, the major sex-specific differences in cardiac structure and function were found to be women having generally smaller ventricular dimensions and volumes, lower left ventricular mass as well as higher biventricular ejection fractions. These results confirm many studies that have established sex-specific reference ranges for quantitative CMR parameters [24, 28, 29]. Likewise, the results of the mentioned studies demonstrated that chamber dimensions decrease with rising age. This seems to be one of the fundamental age-related physiological cardiac adaptations in both sexes. Additionally, by using RV/LV-VR for quantitative assessment, this research revealed that the size of LV and RV declined equally with age in both sexes, represented by a preserved RV/LV-VR. Thus, age as a non-modifiable risk factor may not lead to an excessive workload on a particular single ventricle and a deviation of RV/LV-VR seems to indicate a specific underlying pathology. However, there is currently only limited knowledge about the clinical relevance of RV/LV-VR in CMR. Altmayer et al. demonstrated that the use of RV/LV-VR for diagnostic assessment increases the sensitivity for detecting RV dilatation in patients with pulmonary arterial hypertension [30]. Especially in congenital heart diseases, it was shown that RV/LV-VR seems to be a sex-neutral universal measure for the assessment of RV dilatation and pulmonary regurgitation as illustrated in patients with repaired tetralogy of Fallot [31]. However, the impact of age on the development of RV/LV-VR remains unexplained. Thus, this research, to the best of our knowledge, provides the first relevant CMR insights into the age-associated progression of RV/LV-VR, implicating that this parameter could serve as an age-independent diagnostic measure.
Another essential cardiac alteration was found by an age-associated increase in concentricity (LV-MVR) in both sexes. This ventricular adaptation cannot be explained by an increase in LVM with age, but by the stronger decline of LV end-diastolic volume in relation to LVM. This adjustment reflects rather a remodeling than a hypertrophy. These results confirm the findings of the CMR study by Cheng et al. [17], who investigated age-related ventricular alterations in the large MESA cohort. The study also found that LVM only decreased slightly in the elderly in both sexes, a trend that was similarly observed in this research (insignificant between youngest and oldest age groups), although there were slight differences in the population between MESA and the cohort of this study, which included younger healthy volunteers without traditional cardiac risk factors (e.g., arterial hypertension or hyperlipidemia). These findings were also supported in the longitudinal follow-up trial of the MESA cohort by Eng et al. [32], except for a long-term increase in LVM in men, which has not been reported before. Another healthy but sex-unspecific sample was studied by Kersten et al. [16], who found that LVM and indexed LVM decreased significantly with age while LV-MVR tended to increase in the elderly (insignificantly). Nevertheless, the age-related maintaining or decreasing LVM measured in CMR studies disagrees with echocardiographic trials that reported an age-associated increase in LVM [8, 9]. Representing the currently most important routine imaging modality in cardiology, echocardiography relies on different quantification methods which may influence the quantification of LVM. It was shown that LVM was larger when measured by echocardiography compared to CMR [33,34,35].
There were no clinically relevant sex-specific differences regarding the age-associated development of the above parameters (except for greater LV-MVR and LVM in males). In that regard, these results differ from previous studies that described a higher prevalence of concentric remodeling in females [10, 13, 36, 37]. This may be attributed to a different selection of participants, including the presence of cardiac diseases or risk factors compared to the healthy sample of this research.
Further, a sex-specific difference in the age-related progression of the LV stroke volume index as a determinant of cardiac output could be found. Although there were no significant age-associated changes in LV or RV ejection fractions in both sexes, the LV stroke volume index declined, namely stronger in men compared to women. Literature confirms an age-associated decline of LV stroke volume (index) [16, 17, 32]. Remarkably, the progression of LV-SVI differed slightly depending on whether it was indexed to BSA or H, even though both indices decreased with rising age. Indexed to BSA (weight-dependent), LV-SVI decreased faster compared to LV-SVI indexed to H, especially in males. Generally, the body characteristics of this cohort were comparable to other large populational-based studies [38, 39].
The cellular composition of the ventricular myocardium could provide a possible explanation for the higher biventricular systolic performance and the lower age-related decline in LV-SVI in women. Litviňuková et al. mentioned higher proportions of ventricular cardiomyocytes in women despite an overall lower total mass compared to men [40].
Given the sex-specific crossing point of the age-correlated LV-SVI/BSA trend curves in the years of early menopause onset, one could hypothesize that women entering menopause potentially undergo specific cardiac adaptations that are caused by changes in the sex-hormonal profile. Estrogen was generally associated with numerous cardioprotective mechanisms and higher postmenopausal estrogen levels were associated with a lower risk for cardiac diseases [5, 41,42,43]. Consequently, it is surprising that especially older women with normally decreasing estrogen levels demonstrate preserved and, in some cases, higher systolic LV performance. Further sex hormones can potentially contribute to postmenopausal cardiac changes: Subramanya et al. demonstrated that an androgenic hormone pattern (higher free testosterone, lower sex hormone binding globulin levels) was connected to an increase in LVM and concentricity in postmenopausal women [44]. Future research should aim to further investigate the impact of sex-hormonal changes upon pre- and post-menopausal women regarding the cardiac morphology as well as the ventricular function. Along with the investigation of biological characteristics, the sociocultural background and social identities of patients (gender rather than sex) may also be considered in further research. The scientific and clinical assessment of sociocultural environmental factors such as gender-specific health behavior, lifestyle, physical activity, and nutrition could improve the patient care and provide relevant information about sex- and gender-specific differences in the expression and development of cardiac diseases.
Interestingly, it was shown that a regression of atrial size occurs during aging in correlation with the decrease in ventricular size observed in healthy volunteers [45]. Further, associations with age-related changes in myocardial tissue composition are known as T1-mapping times appear to increase with age, while T2-mapping times tend to decrease [46]. Women additionally demonstrated significantly higher T1-mapping times than men [46, 47]. But, published data in this regard are still conflicting. One could assume that this is related to different study cohorts and techniques. An integrated analysis of atrial and ventricular remodeling including myocardial tissue characterization as well as hemodynamic assessment could be helpful for a deeper understanding of physiological changes in the sex-specific aging heart.
Study limitations
This analysis was based on a retrospective and single-center design. As the identification and inclusion of healthy participants is more challenging with rising age, the participants in each age group could not be equally balanced. A comprehensive analysis of atria, parametric mapping–based myocardial tissue analysis, and phase contrast technique–based flow assessment were not possible as the data were not available in all volunteers. For ethical requirements, a contrast agent could not be applied to healthy volunteers.
Conclusions
By using CMR, this study found several physiological structural and functional heart adaptations during healthy aging. Both women and men showed comparable age-associated ventricular adjustments, as illustrated in the similar increase in concentricity as well as the general decrease of ventricular dimensions. Nevertheless, the female heart cannot just be considered a smaller version of the male’s heart. This study found higher biventricular systolic function and a smaller age–related decline in systolic LV performance in women. To extend knowledge about underlying causes of sex-specific cardiovascular adaptations over a lifetime, additional research will be required.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Roth GA, Johnson C, Abajobir A et al (2017) Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70:1–25. https://doi.org/10.1016/j.jacc.2017.04.052
Savarese G, Becher PM, Lund LH et al (2023) Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 118:3272–3287. https://doi.org/10.1093/cvr/cvac013
Logeart D, Taille Y, Derumeaux G et al (2024) Patterns of left ventricular remodeling post-myocardial infarction, determinants, and outcome. Clin Res Cardiol. https://doi.org/10.1007/s00392-023-02331-z
Keller KM, Howlett SE (2016) Sex differences in the biology and pathology of the aging heart. Can J Cardiol 32:1065–1073. https://doi.org/10.1016/j.cjca.2016.03.017
Yusifov A, Woulfe KC, Bruns DR (2022) Mechanisms and implications of sex differences in cardiac aging. J Cardiovasc Aging 2:20. https://doi.org/10.20517/jca.2022.01
Horn MA, Trafford AW (2016) Aging and the cardiac collagen matrix: novel mediators of fibrotic remodelling. J Mol Cell Cardiol 93:175–185. https://doi.org/10.1016/j.yjmcc.2015.11.005
Redfield MM, Jacobsen SJ, Borlaug BA et al (2005) Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112:2254–2262. https://doi.org/10.1161/CIRCULATIONAHA.105.541078
Vriz O, Pirisi M, Habib E et al (2019) Age related structural and functional changes in left ventricular performance in healthy subjects: a 2D echocardiographic study. Int J Cardiovasc Imaging 35:2037–2047. https://doi.org/10.1007/s10554-019-01665-y
Gebhard C, Stähli BE, Gebhard CE et al (2013) Age- and gender-dependent left ventricular remodeling. Echocardiography 30:1143–1150. https://doi.org/10.1111/echo.12264
Miller RJH, Mikami Y, Heydari B et al (2020) Sex-specific relationships between patterns of ventricular remodelling and clinical outcomes. Eur Heart J - Cardiovasc Imaging 21:983–990. https://doi.org/10.1093/ehjci/jeaa164
Ji H, Kwan AC, Chen MT et al (2022) Sex differences in myocardial and vascular aging. Circ Res 130:566–577. https://doi.org/10.1161/CIRCRESAHA.121.319902
Cheng S, Xanthakis V, Sullivan LM et al (2010) Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation 122:570–578. https://doi.org/10.1161/CIRCULATIONAHA.110.937821
Gori M, Lam CSP, Gupta DK et al (2014) Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail 16:535–542. https://doi.org/10.1002/ejhf.67
Ho JS, Wong JJ, Gao F et al (2023) Adverse cardiovascular and metabolic perturbations among older women: ‘fat-craving’ hearts. Clin Res Cardiol 112:1555–1567. https://doi.org/10.1007/s00392-023-02156-w
Dunlay SM, Roger VL, Redfield MM (2017) Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14:591–602. https://doi.org/10.1038/nrcardio.2017.65
Kersten J, Hackenbroch C, Bouly M et al (2022) What is normal for an aging heart?: a prospective CMR cohort study. J Cardiovasc Imaging 30:202. https://doi.org/10.4250/jcvi.2022.0021
Cheng S, Fernandes VRS, Bluemke DA et al (2009) Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2:191–198. https://doi.org/10.1161/CIRCIMAGING.108.819938
Matthews S, Cook S, Clayton T, et al (2023) Factors affecting women’s participation in cardiovascular research: a scoping review. Eur J Cardiovasc Nurs zvad048. https://doi.org/10.1093/eurjcn/zvad048
Riehle L, Gothe RM, Ebbinghaus J et al (2023) Implementation of the ESC STEMI guidelines in female and elderly patients over a 20-year period in a large German registry. Clin Res Cardiol 112:1240–1251. https://doi.org/10.1007/s00392-023-02165-9
Schulz-Menger J, Bluemke DA, Bremerich J et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 22:19. https://doi.org/10.1186/s12968-020-00610-6
Bluemke DA, Kronmal RA, Lima JAC et al (2008) The relationship of left ventricular mass and geometry to incident cardiovascular events. J Am Coll Cardiol 52:2148–2155. https://doi.org/10.1016/j.jacc.2008.09.014
Miller J, Chaudhry F, Tirgari S et al (2021) Cardiac stroke volume index is associated with early neurological improvement in acute ischemic stroke patients. Front Physiol 12:689278. https://doi.org/10.3389/fphys.2021.689278
Altmayer SPL, Han QJ, Addetia K et al (2018) Using all-cause mortality to define severe RV dilation with RV/LV volume ratio. Sci Rep 8:7200. https://doi.org/10.1038/s41598-018-25259-1
Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B et al (2020) Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 22:87. https://doi.org/10.1186/s12968-020-00683-3
Klinke V, Muzzarelli S, Lauriers N et al (2013) Quality assessment of cardiovascular magnetic resonance in the setting of the European CMR registry: description and validation of standardized criteria. J Cardiovasc Magn Reson 15:55. https://doi.org/10.1186/1532-429X-15-55
Hadler T, Wetzl J, Lange S et al (2022) Introduction of Lazy Luna an automatic software-driven multilevel comparison of ventricular function quantification in cardiovascular magnetic resonance imaging. Sci Rep 12:6629. https://doi.org/10.1038/s41598-022-10464-w
Hadler T, Ammann C, Wetzl J et al (2023) Lazy Luna: extendible software for multilevel reader comparison in cardiovascular magnetic resonance imaging. Comput Methods Programs Biomed 238:107615. https://doi.org/10.1016/j.cmpb.2023.107615
Luu JM, Gebhard C, Ramasundarahettige C et al (2022) Normal sex and age-specific parameters in a multi-ethnic population: a cardiovascular magnetic resonance study of the Canadian Alliance for Healthy Hearts and Minds cohort. J Cardiovasc Magn Reson 24:2. https://doi.org/10.1186/s12968-021-00819-z
Petersen SE, Aung N, Sanghvi MM et al (2017) Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson 19:18. https://doi.org/10.1186/s12968-017-0327-9
Altmayer SPL, Patel AR, Addetia K et al (2016) Cardiac MRI right ventricle/left ventricle (RV/LV) volume ratio improves detection of RV enlargement. J Magn Reson Imaging 43:1379–1385. https://doi.org/10.1002/jmri.25110
Śpiewak M, Małek ŁA, Petryka J et al (2012) Repaired tetralogy of Fallot: ratio of right ventricular volume to left ventricular volume as a marker of right ventricular dilatation. Radiology 265:78–86. https://doi.org/10.1148/radiol.12120051
Eng J, McClelland RL, Gomes AS et al (2016) Adverse left ventricular remodeling and age assessed with cardiac MR imaging: the multi-ethnic study of atherosclerosis. Radiology 278:714–722. https://doi.org/10.1148/radiol.2015150982
Guenzinger R, Wildhirt SM, Voegele K et al (2008) Comparison of magnetic resonance imaging and transthoracic echocardiography for the identification of LV mass and volume regression indices 6 months after mitral valve repair. J Card Surg 23:126–132. https://doi.org/10.1111/j.1540-8191.2007.00558.x
Breitenbach I, Harringer W, Tsui S et al (2012) Magnetic resonance imaging versus echocardiography to ascertain the regression of left ventricular hypertrophy after bioprosthetic aortic valve replacement: results of the REST study. J Thorac Cardiovasc Surg 144:640–645.e1. https://doi.org/10.1016/j.jtcvs.2011.11.017
Armstrong AC, Gjesdal O, Almeida A et al (2014) Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Echocardiography 31:12–20. https://doi.org/10.1111/echo.12303
Krumholz HM, Larson M, Levy D (1993) Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol 72:310–313. https://doi.org/10.1016/0002-9149(93)90678-6
Treibel TA, Kozor R, Fontana M et al (2018) Sex dimorphism in the myocardial response to aortic stenosis. J Am Coll Cardiol Img 11:962–973. https://doi.org/10.1016/j.jcmg.2017.08.025
Peter RS, Fromm E, Klenk J et al (2014) Change in height, weight, and body mass index: longitudinal data from Austria. Am J Hum Biol 26:690–696. https://doi.org/10.1002/ajhb.22582
Drøyvold WB, Nilsen TIL, Krüger Ø et al (2006) Change in height, weight and body mass index: Longitudinal data from the HUNT study in Norway. Int J Obes 30:935–939. https://doi.org/10.1038/sj.ijo.0803178
Litviňuková M, Talavera-López C, Maatz H et al (2020) Cells of the adult human heart. Nature 588:466–472. https://doi.org/10.1038/s41586-020-2797-4
Piro M, Della Bona R, Abbate A et al (2010) Sex-related differences in myocardial remodeling. J Am Coll Cardiol 55:1057–1065. https://doi.org/10.1016/j.jacc.2009.09.065
Vitale C, Fini M, Speziale G, Chierchia S (2010) Gender differences in the cardiovascular effects of sex hormones. Fundam Clin Pharmacol 24:675–685. https://doi.org/10.1111/j.1472-8206.2010.00817.x
Zhao D, Guallar E, Ouyang P et al (2018) Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 71:2555–2566. https://doi.org/10.1016/j.jacc.2018.01.083
Subramanya V, Zhao D, Ouyang P et al (2018) Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 108:37–44. https://doi.org/10.1016/j.maturitas.2017.11.006
Funk S, Kermer J, Doganguezel S et al (2018) Quantification of the left atrium applying cardiovascular magnetic resonance in clinical routine. Scand Cardiovasc J 52:85–92. https://doi.org/10.1080/14017431.2017.1423107
Roy C, Slimani A, de Meester C et al (2017) Age and sex corrected normal reference values of T1, T2 T2* and ECV in healthy subjects at 3T CMR. J Cardiovasc Magn Reson 19:72. https://doi.org/10.1186/s12968-017-0371-5
Cavus E, Schneider JN, Bei der Kellen R et al (2022) Impact of sex and cardiovascular risk factors on myocardial T1, extracellular volume fraction, and T2 at 3 Tesla: results from the population-based, Hamburg City Health Study. Circ Cardiovasc Imaging 15(9):e014158. https://doi.org/10.1161/CIRCIMAGING.122.014158
Acknowledgements
Special thanks go to all members of the CMR working group for their helpful input, particularly to the members who screened and recruited the volunteers in previous studies. Another special mention is dedicated to the CMR technicians Kerstin Kretschel, Denise Kleindienst, and Martina Kohla for performing the CMR examinations as well as to the study nurses Annette Köhler and Elke Nickel for their beneficial assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. T.H. receives funding from the German Research Foundation (GRK2260, BIOQIC). L.D.K. receives funding from the German Heart Foundation (Kaltenbach Doktorandenstipendium). J.S.M. holds institutional grants from the Charité University Medicine Berlin, Germany. None of the funding interfered with the research or was influenced by them. All other authors have reported that there are no relationships relevant to the content of this publication that are required to be disclosed.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript and have contributed significantly to the conception and interpretation of data as well as drafting the manuscript itself: study concepts and design: Leonhard Grassow, Jan Gröschel, Jeanette Schulz-Menger; data acquisition, analysis, and interpretation: all authors; literature research: Leonhard Grassow, Jan Gröschel, Leo Dyke Krüger, Jeanette Schulz-Menger; statistical analysis: Leonhard Grassow, Jan Gröschel, Jeanette Schulz-Menger; manuscript drafting or revision: all authors; approval of the final version of submitted manuscript: all authors; agrees to ensure any questions related to the work are appropriately resolved: all authors.
Corresponding author
Ethics declarations
Ethical statements
All data acquisition and subject recruitment were approved by the ethics committee of the Charité University Medicine Berlin, Germany, and were subject to the ethical standards of the institution and the Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the studies.
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grassow, L., Gröschel, J., Saad, H. et al. Sex-specific structural and functional cardiac remodeling during healthy aging assessed by cardiovascular magnetic resonance. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02430-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02430-5