Abstract
Background
Severe tricuspid regurgitation (TR) is associated with chronic volume overload and right ventricular remodeling (RVR). Transcatheter tricuspid valve repair (TTVr) reduces TR and can improve quality of life (QoL), but the role of preprocedural RVR on TTVr outcomes remains unclear.
Aims
To investigate the role of RVR on outcomes after TTVr for severe TR.
Methods
Consecutive patients undergoing TTVr (61% edge-to-edge vs. 39% direct annuloplasty) for severe TR were retrospectively compared by preexisting RVR which was defined as dilation of RV mid-level diameter (> 35 mm) according to guidelines. QoL was evaluated using NYHA class, Minnesota Living with Heart Failure Questionnaire (MLHFQ), 36-Item Short Form Health Survey (SF-36), and 6-min walking distance (6MWD) 1-month after TTVr. Mid-term mortality and heart failure (HF) hospitalization were assessed through 1 year.
Results
RVR was present in 137 of 223 patients (61%). Symptoms and QoL improved equally in both groups: ≥ 1 NYHA class (57% vs. 65% of patients with vs. without RVR, respectively), 6MWD (36% vs. 34%), MLHFQ (81% vs. 69%), and SF-36 (68% vs. 65%) improvement. One-year mortality and HF hospitalization were significantly higher in patients with RVR (24% and 30%, respectively) than in patients without (8% and 13%, both p < 0.05). In multivariable analysis, RVR was independently associated with mortality (HR 2.3, 95%CI (1.0–5.0), p = 0.04) and the combined endpoint of mortality or rehospitalization (HR 2.0, 95%CI (1.1–3.8), p = 0.03).
Conclusions
TTVr was associated with significant QoL improvement after 1 month, irrespective of RVR. Despite increased mortality and rehospitalization for heart failure, TTVr in the presence of RVR still provides substantial symptomatic benefit for patients with severe TR.
Graphical abstract
Role of preexisting right ventricular remodeling (RVR) in symptoms and prognosis after transcatheter tricuspid valve repair (TTVr).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tricuspid regurgitation (TR) is one of the most prevalent valvular diseases in the elderly population and significantly worsens clinical outcomes and survival in patients with heart failure [1]. With an aging population, the prevalence of TR is expected to increase in the future [2]. Therefore, there is intensive debate about the necessity of treatment options that might impact clinical outcomes and at the same time provide sufficient safety. As conventional surgery for isolated severe TR has a significant perioperative risk and has not been proven to improve survival compared to medical management alone, treatment of symptomatic severe isolated TR has shifted towards a conservative approach for the majority of patients [3, 4]. Over the last few years, several transcatheter technologies have been developed to address the need for alternative treatment options for high-risk populations that are not eligible for surgery [5, 6]. First steps have been taken by proving the feasibility and safety of the procedure [7].
Nevertheless, the functional status of patients following transcatheter tricuspid valve repair (TTVr) regarding symptom burden and quality of life (QoL) remains poorly studied. Health-related QoL is a strong predictor of clinical outcome and all-cause mortality in patients with heart failure [8]. Hence, a major goal of TTVr treatment is to increase QoL in patients with severe symptoms [9]. It is fundamental to acknowledge TR as a heterogeneous valve disease with distinguishable phenotypes [10, 11]. Chronic volume overload occurring in severe TR culminates in right ventricular (RV) dilation and dysfunction, which has been shown to negatively affect prognosis in patients with heart failure [10, 12]. In patients undergoing isolated tricuspid valve (TV) surgery, preoperative RV remodeling (RVR) can be identified as a predictor of poor outcomes and has been associated with decreased overall survival [13, 14]. However, a systematic analysis of the TR phenotype and disease stage, which are both associated with RVR, is lacking. As TTVr is gaining importance, it is essential to evaluate the effect of preprocedural RVR in the transcatheter context. Therefore, this study aimed to analyze the impact of TTVr on procedural success, various measures of functional capacity and QoL, and mid-term clinical outcomes in the context of the presence or absence of preprocedural RVR.
Methods
Study population
This study was conducted at the Heart Centre at the University of Cologne. The study protocol conformed to the 1975 Declaration of Helsinki and is in line with the established Ethical Guidelines for Epidemiological Research. Consecutive patients who underwent TTVr and signed an informed consent form at our high-volume referral center were included in the study. We retrospectively studied patients between January 2017 and October 2020 and prospectively enrolled patients between October 2020 and December 2022. Despite receiving optimal medical therapy according to current guidelines, all patients were classified as New York Heart Association (NYHA) functional class ≥ II. The entire study population underwent discussion in the interdisciplinary heart team conference with a concordant decision on interventional treatment with transcatheter edge-to-edge repair or transcatheter direct annuloplasty via Cardioband implantation. This study was approved by the local ethics committee of the University of Cologne.
Echocardiographic assessment
All patients underwent transthoracic echocardiographic assessment one day prior to the procedure. All echocardiograms were evaluated by a trained cardiologist following the current guidelines for echocardiographic assessment of valve regurgitation [15, 16]. In order to capture the severity of TR and to study the reduction in TR properly, we used a five-class grading scheme to quantify TR grade, previously proposed by Hahn and Zamorano [17]. The TR grade was classified as none or mild (I°), moderate (II°), severe (III°), massive (IV°), and torrential (V°) using qualitative (color flow jet), semiquantitative (vena contracta, systolic flow reversal in hepatic veins), and quantitative (effective regurgitant orifice area, regurgitant volume) parameters according to current guidelines. TR was secondary in all of the cases. Furthermore, right heart catheterization was performed to assess pulmonary hypertension (PH) prior TTVr. PH was classified in precapillary, isolated postcapillary, and combined pre- and postcapillary PH according to current guidelines [18]. Chronic thromboembolic pulmonary hypertension (CTEPH) was excluded via computed tomography or ventilation/perfusion scan in all patients with clinical suspicion and precapillary PH. Patients with diagnosed CTEPH were treated with specific therapy and not with TTVr and were therefore excluded from this study.
RV size was evaluated by measuring the RV cavity area and RV cavity diameter in two-dimensional RV-focused apical four-chamber view. RVR was defined as dilation of the RV at the mid-level (> 35 mm diameter, according to current guidelines) [15]. The RV basal diameter was not considered a distinct indicator of RVR, as it can be dilated along with the TV annulus in isolated RA enlargement [19]. RV dysfunction was defined as a TAPSE < 17 mm or FAC < 35%. Echocardiographic evaluation was repeated at discharge and at follow-up, approximately 4–6 weeks after the procedure.
Evaluation of the functional capacity and QoL
We obtained a thorough functional status for all prospective patients. Functional capacity and QoL assessments were performed by a trained medical student who was blinded to procedural and echocardiographic results. Evaluation was incorporated into the clinical routine prior to TTVr as part of the prospective analysis of our study. Six-min walking distance (6 MWD) was measured [20]. Acknowledging the occurrence of peripheral edema as a major cardinal symptom of right heart failure and TR, we quantitatively analyzed the occurrence of edema [21, 22]. The grading of peripheral edema used in this study is a modification of the classic clinical assessment described by Seidel et al. [23]. We classified three grades of edema according to severity, as measured by physical examination. Grade I was defined as pitting edema with up to 2 mm of depression and immediate rebound. Grade II was defined as pitting edema with 2–4 mm of depression and rebounding within 10–25 s. Grade III was defined as pitting edema with more than 4 mm of depression and rebound in more than 1 min. The Minnesota Living with Heart Failure Questionnaire (MLHFQ) was used to assess QoL [24]. The MLHFQ contains 21 questions (− 0–5 points for each question) representing physical, mental, and social components that can be affected by heart failure (HF). Additionally, a short version of the Short Form-36 (SF-36) health survey questionnaire containing 12 items reflecting the original 8 subscales of the SF-36 was conducted [25]. Item scores were coded, totalized, and transformed on a scale from 0 (worst health) to 100 (best health), covering functional status and general well-being as well as physical and mental health. Assessment was conducted during hospitalization before the procedure and at follow-up 4–6 weeks after the procedure.
TTVr procedures
The procedures were performed using MitraClip, TriClip (Abbott Vascular), PASCAL P10, PASCAL Ace (Edwards Lifesciences) devices, or Cardioband device (Edwards Lifesciences), as previously described [6, 26, 27]. As direct annuloplasty is more complex than TEER, both regarding patient screening including computed tomography as well as longer and more complex procedure, TEER is the first treatment choice in daily clinical practice. In general, every patient which showed a tricuspid valve anatomy with difficulty in leaflet grasping (e.g., extremely large coaptation gap, short leaflets, or multiple-scallop leaflets) was evaluated for direct annuloplasty. Technical success was defined as successful device implantation without conversion to emergent TV surgery or re-intervention and absence of mortality, as well as successful deployment and correct positioning of the device. Additionally, procedural success was confirmed if the postprocedural TR grade at discharge was moderate or less (≤ II°).
Endpoints
Next to TR grade, the presence and grade of peripheral edema, NYHA functional class, and QoL scores (for the prospective patient population) were assessed at 4–6 weeks following TTVr. Preprocedural RVR was assessed in all patients to analyze its impact on clinical and functional outcomes. We captured all-cause mortality, occurrence of first rehospitalization for decompensated HF, and occurrence of repeat TV intervention as mid-term clinical outcomes by contacting patients’ general physicians and using register queries.
Statistical analysis
Characteristics are expressed as mean ± standard deviation (SD) for continuous variables if normally distributed and as median (IQR) if not normally distributed. Normal distribution was tested for all variables using the Shapiro–Wilk test. Categorical data were presented as counts (percentages). Differences in baseline characteristics between patients with and without preprocedural RVR were determined by applying the independent t-test if normally distributed and the Mann–Whitney U test if not normally distributed for continuous variables. Differences in categorical variables were determined using Pearson’s χ2 test. Changes in NYHA functional class, TR grade, grade of peripheral edema, 6 MWD, and MLWFQ were studied using the Wilcoxon matched pairs signed rank test. Changes in the occurrence of peripheral edema were analyzed using the McNemar’s test. Changes in SF-36 scores were analyzed by applying a paired t-test. Kaplan–Meier plots were used to depict the event curves of the patients with and without RVR. Furthermore, we used Cox regression models to investigate the impact of preprocedural RVR on mid-term clinical outcomes, including all-cause mortality, HF hospitalization, and the combined endpoint. Risk was expressed as hazard ratio (HR), 95% confidence interval (CI), and p-value. Univariable Cox regression analysis was conducted for all baseline variables. Variables with a p < 0.05 in the univariable Cox regression analysis were selected for adjustment in a multivariable Cox regression model. For redundant variables, such as echocardiographic severity (VC, EROA, and RV), only one was considered for multivariable analysis. Natriuretic peptides were not included in the regression analysis because of multiple confounders (renal dysfunction and obesity). The multiparametric scores (EuroSCORE II, TRI-SCORE) were also not considered for multivariable analysis because the individual components of the scores were already included. A two-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics version 28 (IBM, Armonk, NY, USA).
Results
Study population
A total of 223 patients who underwent TTVr were included in the study. Eighty-two patients were retrospectively analyzed, and 141 patients underwent prospective evaluation. The baseline clinical and echocardiographic characteristics are shown in Table 1. The median age was 80 years (IQR, 75–83), and 69% of the patients were female. The median European System for Cardiac Operative Risk Evaluation score II (EuroScore II) was 4.1% (IQR, 2.8–7.2%), and the mean TRI-SCORE was 6.3 ± 1.9 points. All patients had right heart failure and were symptomatic, with signs and symptoms of at least one of the following: chest discomfort, breathlessness, palpitations, and edema. Most patients were severely symptomatic at admission, with 85% presenting with NYHA functional class III or IV.
A total of 137 patients (61%) had preprocedural RVR, and 86 patients (39%) had no RVR. Patients with RVR were significantly more male (p < 0.001). Both groups were highly symptomatic, with a similar distribution of NYHA functional class grading. Patients with RVR received a significantly (p < 0.001) higher dose of loop diuretics at baseline (60 mg/day, IQR, 20 mg/day to 100 mg/day with RVR, vs. 30 mg/day, IQR, 20 mg/day to 50 mg/day without RVR; p < 0.001) than patients without RVR. The occurrence of peripheral edema did not differ between the groups. Prior to TTVr, patients with RVR had more frequently a history of hospitalization for HF (67% vs. 50%, p = 0.01), diabetes mellitus (31% vs. 14% of patients, p = 0.003), prior myocardial infarction (7% vs. 1%, p = 0.039), higher NT-proBNP level (median 2,627 vs. 1,368 ng/l, p < 0.001), and higher TRI-SCORE (6.5 ± 1.9 vs. 6.0 ± 1.8, p = 0.026). A detailed comparison of the baseline characteristics of the patients with and without RVR is presented in Table 1.
Echocardiographic assessment
The TR grade was significantly higher in patients with RVR compared with those without RVR. Forty-seven percent vs. 33% of patients with vs. without RVR, respectively, presented a TR grade of massive or torrential (p = 0.008). Patients with RVR also showed higher values for all parameters of quantitative TR grade measurement (EROA 0.62 vs. 0.46 cm2, p = 0.007; RV 50 vs. 39 ml, p < 0.001; VC 12 vs. 9 mm, p < 0.001). The frequency of pulmonary hypertension did not differ among the groups (76% vs. 69%, p = 0.231). The mean pulmonary artery pressure measured in right heart catheterization was higher in patients with RVR (31 vs. 27 mmHg, p = 0.049). Additionally, the mean pulmonary artery wedge pressure was higher in patients with RVR (19 vs. 16 mmHg, p = 0.006). The etiology of pulmonary hypertension showed marked differences between the two groups, with patients with RVR having a distribution of 16% with precapillary pulmonary hypertension, 55% with isolated postcapillary hypertension, and 29% with combined precapillary and postcapillary hypertension, whereas those without RVR had percentages of 30%, 33%, and 37%, respectively, for the aforementioned classifications (p = 0.036). Whereas the mean TAPSE was not statistically different among groups (17 mm vs. 18 mm, p = 0.126), reduced FAC (< 35%) was more frequent in patients with RVR (68% vs. 27%, p < 0.001). Hepatic systolic flow reversal was more frequent in patients with RVR (82% vs. 62%, p = 0.005). A detailed comparison of the baseline echocardiographic assessments of the patients with and without RVR is presented in Table 1.
Procedural results of TTVr
Cardioband implantation was performed in 87 patients (39%), and MitraClip in the tricuspid position was used in 11 patients (5%). TriClip devices were used in 49 patients (22%). PASCAL devices were used in 76 patients (34%). Technical success was achieved in 209 (94%) patients. In seven patients (3%), TTVr was unsuccessful due to unfavorable anatomical, technical, or procedural circumstances, and the device was removed without complications. Procedural success, defined as a moderate or less TR at hospital discharge, was achieved in 64% of the attempted procedures. None of the patients was lost to follow-up at 1 month regarding vital status. Seven patients (3%) died postprocedural or during the 1-month follow-up period.
There was no difference regarding the presence of RVR at baseline between patients undergoing edge-to-edge repair and patients undergoing direct annuloplasty (59% vs. 65%, p = 0.316). The technical success rate was higher in patients without RVR (98% vs. 91% with RVR, p = 0.054). All-cause mortality rate within 1 month of TTVr was higher in patients with RVR (p = 0.033). Procedural success rate was higher in the absence of RVR (78% vs. 55%, p < 0.001). There were no differences in procedural aspects or complications between TTVr devices. Head-to-head comparison of procedural information and major complications associated with the two different TTVr strategies are shown in the supplemental file. A detailed comparison of procedural results is presented in Table 2. TR grade at discharge was significantly lower in patients without RVR (p = 0.004). In comparison with TR grade at baseline, the TR grade at 1-month follow-up was significantly reduced regardless of RVR. In comparison with TR grade at discharge, the TR grade at the 1-month follow-up did not change in either group. Fifty-six percent vs. 78% of patients with vs. without RVR presented with a NYHA class of ≤ II at discharge (p = 0.004).
Functional capacity and QoL
Overall, at the 1-month follow-up, the rate of NYHA functional class III/IV reduced from 83% at baseline to 34% at the 1-month follow-up (p < 0.001). Grade III peripheral edema decreased from 10% at baseline to 4% at the 1-month follow-up (p = 0.003). The mean 6 MWD increased from baseline to 1-month follow-up (p < 0.001), with 35% of patients showing a clinically relevant improvement of 50 m or more. The median MLHFQ score decreased from baseline to 1-month follow-up (p < 0.001), with 76% of the patients presenting with a clinically relevant improvement of at least 5 points. Additionally, the mean SF-36 score increased from baseline 1-month follow-up (p < 0.001), with 67% of patients showing a clinically relevant improvement of at least 2.5 points.
Significant improvement in NYHA functional class was observed in patients regardless of RVR. At the 1-month follow-up, NYHA functional class improved by at least one grade in 57% and 65% of the patients with and without RVR, respectively (p = 0.262). The rate of NYHA functional class III/IV decreased from 84 to 39% vs. from 82 to 27%, at the 1-month follow-up in patients with and without RVR, respectively (p < 0.001).
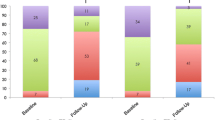
The occurrence of peripheral edema decreased from 59 to 53% vs. from 55 to 37% at the 1-month follow-up in patients with and without RVR, respectively. A significant reduction in the occurrence of peripheral edema was observed only in patients without RVR (p = 0.015). Patients with and without RVR showed a significant decrease in the grade of peripheral edema, with a decrease of at least one grade in 30% of patients with RVR and 31% of patients without RVR. Changes in NYHA class and the status of peripheral edema are illustrated in Fig. 1.
A Comparison of 1-month changes in NYHA functional class after TTVr in patients with and without RVR. B Comparison of 1-month changes in the occurrence of peripheral edema after TTVr in patients with and without RVR. C Comparison of 1-month changes in grade of peripheral edema after TTVr in patients with and without RVR
In patients with RVR, the mean 6 MWD increased from 262 m ± 109 at baseline to 307 m ± 110 at the 1-month follow-up (p < 0.001), whereas in patients without RVR, the mean 6 MWD increased from 280 m ± 104 to 311 m ± 98 at the 1-month follow-up (p = 0.016). Thirty-six percent and 34% showed a clinically relevant increase in the 6 MWD of at least 50 m for patients with and without RVR, respectively, as shown in Fig. 2.
Both groups showed significant improvement in QoL at the 1-month follow-up compared with the preprocedural baseline. In patients without RVR, the median MLHFQ score decreased from 41 (IQR, 24–49) at baseline to 27 (IQR, 17–39) at the 1-month follow-up (p < 0.001), and 69% showed an improvement of at least 5 points. In comparison, the mean MLHFQ score in patients with RVR decreased from 41 (IQR, 29–54) at baseline to 27 (IQR, 15–41) at the 1-month follow-up (p < 0.001), and 81% showed an improvement of at least 5 points. Additionally, the mean SF-36 score in patients without RVR improved from 51 ± 22% at baseline to 62 ± 19% at the 1-month follow-up (p < 0.001), with 65% of the patients showing an improvement of at least 2.5 points. The mean SF-36 score in patients with RVR increased from 50 ± 21% at baseline to 61 ± 21% at the 1-month follow-up (p < 0.001), with 68% of patients showing an improvement of at least 2.5 points. Functional capacity and QoL did not differ between devices. The changes in the MLHFQ and SF-36 scores are shown in Fig. 2.
QoL data were available for 35 patients at 12 months. NYHA functional class was available for 59 patients at 12 months. 6MWD was available for 30 patients at 12 months. Median MLHFQ scores at 12 months were 21 (IQR, 11–39) and 32 (IQR, 19–37) in patients with and without RVR, respectively. The mean SF-36 scores at 12 months were 65 ± 20% and 53 ± 19% in patients with and without RVR, respectively.
The rate of NYHA functional class III/IV at 12 months was 36% and 32% in patients with and without RVR, respectively. The incidence of peripheral edema at 12 months was 33% and 30% in patients with and without RVR, respectively. The mean 6MWD at 12 months was 324 ± 148 and 309 ± 108 in patients with and without RVR, respectively.
Mid-term clinical outcomes
Mid-term follow-up data were available after 463 ± 403 days (median, 374 days; IQR, 156–607). Six-month follow-up was available in 84% of patients, and 12-month follow-up was available in 68% of patients. Information about death after TTVr was available in all patients, and information about rehospitalization due to HF after TTVr was available in 76% of patients.
One year after the procedure, 19% of the patients died, 25% were readmitted to the hospital due to decompensated heart failure, and one patient had to undergo repeat TTVr because of severe residual TR.
All-cause mortality 1 year after TTVr was significantly higher in patients with RVR (24% vs. 8%, p = 0.013), and RVR was associated with a HR of 2.6 (95% CI, 1.2–5.6) for all-cause mortality (p = 0.016). One-year heart failure (HF) rehospitalization after TTVr was significantly higher in patients with RVR (30% vs. 13%, p = 0.045), and RVR was associated with a HR of 2.4 (95% CI, 1.1–5.7) for rehospitalization due to HF after TTVr (p = 0.038). The combined endpoint of 1-year mortality or 1-year rehospitalization due to HF after TTVr was seen significantly more often in patients with RVR (44% vs. 21%, p = 0.012), and RVR was associated with a HR of 2.4 (95% CI, 1.3–4.5) for the combined endpoint of mortality or rehospitalization due to HF after TTVr (p = 0.005). Outcomes between devices were not different. The need for repeat TTVr was not associated with RVR. The Kaplan–Meier curves of all-cause mortality and rehospitalization due to HF are shown in Fig. 3.
In the multivariable analysis, RVR remained to be independently associated with mid-term mortality (HR 2.3, 95%CI (1.0–5.0), p = 0.042) and the combined endpoint (HR 2.0, 95%CI (1.0–3.8), p = 0.027). Mid-term clinical outcomes did not differ between devices. A detailed comparison of the uni- and multivariable analysis is presented in Table 3.
Discussion
This study analyzed procedural success, functional capacity, and QoL, as well as mid-term clinical outcomes in patients with symptomatic HF due to TR following TTVr regarding preprocedural RVR. The main findings can be summarized as follows: (i) TTVr is a safe and effective treatment option in patients regardless of RVR; (ii) TTVr resulted in significant QoL improvement at 1 month; (iii) patients with RVR showed higher 1-year mortality and HF hospitalization; and RVR was independently associated with all-cause mortality and the combined endpoint of mortality or rehospitalization after TTVr.
Importance of preprocedural RV remodeling in TTVr
It is of fundamental importance to acknowledge functional TR as a heterogeneous valve disease with distinguishable phenotypes regarding its pathophysiology [10, 11]. The two main phenotypes of functional TR that must be differentiated are atrial functional TR, in which the development of regurgitation is primarily due to right atrial dilation, and ventricular functional TR, in which the main mechanism leading to regurgitation is dilation and remodeling of the right ventricle with consequent tethering of valve leaflets [11, 28]. However, in the literature, the definitions of atrial TR vary. Previous definitions of atrial TR mainly based on clinical criteria, whereas there is currently a trend of using echocardiographic parameters to classify TR etiology [1, 11, 29]. For reasons of clinical utilization and with respect to the primary pathophysiological processes, we decided to approximate the entity of atrial TR as TR in the absence of RVR, as proposed by Prihadi et al. in 2019 [30]. Consequently, RVR was defined as dilation of the RV at the mid-level (> 35 mm diameter, according to current guidelines) [15]. A thorough multiparametric and multimodal investigation of the heterogeneous TR population is sophisticated and cumbersome to apply on a daily basis. To this end, the definition was based on only one variable to provide a simple and hands-on parameter for risk assessment of patients undergoing TTVr in everyday clinical practice. The RV basal diameter was not considered a distinct indicator of RVR, as it can be dilated along with the TV annulus in isolated RA enlargement [19].
As patients with progressive RVR showed significant functional abnormalities, these patients had more frequently reduced FAC (< 35%) (68% vs. 27%, p < 0.001). The role of RVR on outcomes of patients undergoing treatment of TR is largely unknown. TR grade at baseline was significantly higher in patients with RVR than in those without RVR (p = 0.008). Nevertheless, we acknowledge the relationship between RVR and severity of TR as part of a continuous vicious cycle in which causality cannot be completely clarified, since RVR influences TR severity and vice versa [31]. However, the manifestation of RVR is an important finding that shows an advanced and/or adverse clinical stage in patients with TR. Considering surgery as the only treatment of TR until recently, Calafiore et al. reported an HR of 6.47 (95% CI, 3.88–10.77) for patients with RVR who underwent isolated TV surgery (vs. HR of 2.6 in our study), which confirmed that RVR is a risk factor for lower survival [13]. In this study, RVR was defined as either RV dilation (basal end-diastolic diameter > 42 mm and/or mid-level diameter > 35 mm) or dysfunction (tricuspid annular plane systolic excursion < 16 mm and/or tissue Doppler S-velocity < 10 cm/s) [13]. Consequently, the comparability of the results with those of our study may be limited because of differences in the definition of RVR. However, the patient population with RVR defined by our approach (dilation of RV at mid-level with diameter > 35 mm) was included in their definition of RVR. The fact that RVR has a larger impact on outcomes after surgery compared to TTVr has numerous explanations. Because right heart failure is accelerated by myocardial ischemia after cardiopulmonary bypass and suboptimal myocardial protection during surgery, the increased strain on the right heart during cardiac surgery compared with TTVr might be an important reason for worse surgical outcomes in patients with preexisting RVR [32]. Another contributing factor of worsening right heart function with cardiac surgery might be the need for excessive blood transfusions and the subsequent increase in right ventricular preload, which further worsens right ventricular function with RVR [32]. Therefore, the reasons mentioned above might be seen as advocacy for the transcatheter approach in patients with RVR, even though RVR is still associated with an increase in mortality compared with patients without RVR.
Technical and procedural success of TTVr
The present study confirmed that TTVr is a safe procedure in a highly symptomatic patient population, which showed a substantial surgical risk profile and was therefore not eligible to undergo a surgical approach for TV repair. The rate of technical success we observed was high and comparable to that of recent studies conducted in a similar patient population undergoing TTVr [33]. High technical success rates were observed in both surveyed phenotypes of TR, and no significant differences were observed between patients with and without preprocedural RVR. Interestingly, the vast majority of unsuccessful device implantations occurred in patients with RVR.
The TR degree was significantly reduced after TTVr in patients with and without prior RVR at the 1-month follow-up. The prevalence of procedural success was significantly higher in patients without RVR than in those with RVR (55% vs. 78%, p < 0.001). This is an important finding which could be explained by greater degree of TR and TV deformation including larger valve diameter with larger coaptation gap due to RV dilation and consecutively more challenging anatomy for catheter-based treatments.
In comparison to the considerably less complex mitral valve anatomy, Yoshida et al. reported no differences in procedural success and residual regurgitation between patients with atrial functional MR and those with ventricular MR undergoing percutaneous edge-to-edge mitral valve repair [34]. Nevertheless, we acknowledge MR and TR as separate valve diseases that differ in etiology and clinical impact on heart failure progression. Therefore, the results of TMVr regarding ventricular remodeling are not directly transferrable to TTVr and are comparable only to a limited extent.
Functional capacity and QoL
The present study captured functional capacity and QoL by analyzing NYHA functional class, occurrence, and grade of peripheral edema, and 6 MWD, as well as changes in MLHFQ and SF-36 scores. There was a significant improvement in NYHA functional class at the 1-month follow-up compared with baseline, with 66% of patients having NYHA class I or II, which is promising. Smaller series have shown previously that NYHA class can be reduced after TTVr, albeit with off-label use of edge-to-edge device [35]. Here, we did not see a device-related difference in outcomes. Additionally, there was a significant improvement in both surveyed phenotypes, which might indicate a favorable short-term functional outcome of TTVr, regardless of prior RVR or even reversibility of RVR by TTVr. As peripheral edema is a cardinal symptom of TR, we observed changes in the occurrence and grade of edema before and after TTVr. We observed a significant improvement in peripheral edema grade from baseline to 1-month follow-up in both groups, which might imply that even though peripheral edema was still present in some patients, right heart function improved, and volume overload decreased after TTVr in the majority of cases. This is further affirmed by the fact that no up-titration in loop diuretics doses was observed at the 1-month follow-up compared with baseline; therefore, we cannot attribute the edema regression to escalated medical therapy. Nevertheless, peripheral edema was completely absent in 47% and 63% of 1-month follow-up for patients with and without RVR, respectively (p = 0.029), which might indicate that RVR has a negative impact on right heart function after TTVr. Moreover, we assessed functional capacity by comparing the 6 MWD in all prospectively recruited patients. Overall improvement of 6 MWD was + 39 m (significantly higher compared with baseline, p < 0.001). Small reports on edge-to-edge devices only showed a mean 6 MWD increase of approximately 33 m [36]. However, a clinically relevant increase of at least 50 m was present in only one-third of patients and was not different between groups. Furthermore, the present study demonstrated that TTVr improved QoL by showing significant improvement in MLHFQ and SF-36 scores at the 1-month follow-up, which is comparable to previous studies investigating QoL after TTVr and TMVr, respectively [33, 37]. In addition, we could complement the analysis by showing that there is an improvement in QoL after TTVr regardless of RVR presence.
In summary, our results support the conclusion that TTVr improves functional capacity and QoL in patients with heart failure. Moreover, it seems to do so without regard to the presence of RVR, even though there might be indications of at least some impact of RVR on right heart function after TTVr.
Mid-term clinical outcomes
Despite the refinement of functional capacity and QoL, the association with mid-term survival is still largely unknown in the context of TTVr. The only randomized trial so far in this field showed no survival benefit after edge-to-edge TR repair [10]. Compared with this study, our overall observed rate of all-cause mortality at the 12-month follow-up was higher (19%), which can probably relate to a sicker population and higher prevalence of severe comorbidities (more patients with diabetes, kidney disease, chronic obstructive lung disease, and NYHA class III/IV for instance). On the contrary, other early experience observational studies had similar one-year outcomes after TTVr and TMVr [38, 39].
Considering the role of maladaptive RVR in unfavorable clinical outcomes, concerns have been raised in TMVr [40]. Similar results were observed in patients who underwent TV surgery, as mentioned earlier [13]. Additionally, we observed that patients with RVR showed higher prevalence of the combined endpoint of one-year mortality and HF hospitalization. Most importantly, RVR was independently associated with all-cause mortality and the combined endpoint of mortality or rehospitalization after TTVr. Consequently, even though our data suggest an improvement in functional capacity and quality of life regardless of TR etiology already after 1 month, mid-term clinical outcomes and survival in particular seem to be negatively affected by the presence of preprocedural RVR. This hypothesis-generating finding should be further analyzed in larger controlled studies to confirm the causality of RVR in the prognosis of patients after TTVr.
Limitations
Several limitations should be acknowledged. First, this is an observational single-center study, and our results are hypothesis-generating. Second, imaging quality was not high enough in all patients for 3D image acquisition; thus, more elaborate echo measures on RV anatomy and function are lacking. Third, there was no adjudication committee for the reported clinical outcomes. Fourth, differentiation of TR phenotype did not lie in the scope of this manuscript as our goal was to provide a hands-on parameter for risk assessment in everyday clinical practice. Nevertheless, further research in this direction should be promoted to fully elucidate the complex pathophysiology and associated risk in patients with TR. Finally, we analyzed not only one but several devices which have been implanted in consecutive patients. However, outcomes between devices were not different, and additionally reporting real-world outcomes should be considered as a strength of this study.
Conclusion
TTVr resulted in significant QoL improvement already after 1 month, irrespective of RVR. Patients with RVR showed higher 1-year mortality and HF hospitalization rates; RVR was independently associated with all-cause mortality and the combined endpoint of mortality or rehospitalization after TTVr.
Abbreviations
- 6 MWD:
-
Six-minute walking distance
- CI:
-
Confidence interval
- EROA:
-
Effective regurgitant orifice area
- FAC:
-
Fractional area change
- HF:
-
Heart failure
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- MLHFQ:
-
Minnesota Living with Heart Failure Questionnaire
- NYHA:
-
New York Heart Association
- QoL:
-
Quality of life
- RV:
-
Right ventricle/right ventricular
- RVR:
-
Right ventricular remodeling
- SF-36:
-
36-Item Short Form Health Survey
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TR:
-
Tricuspid regurgitation
- TTVr:
-
Transcatheter tricuspid valve repair
- TV:
-
Tricuspid valve
- VC:
-
Vena contracta
References
Fender EA, Zack CJ, Nishimura RA (2018) Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart 104:798–806
Singh JP, Evans JC, Levy D et al (1999) Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 83:897–902
Axtell AL, Bhambhani V, Moonsamy P et al (2019) Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol 74:715–725
Zack CJ, Fender EA, Chandrashekar P et al (2017) National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 70:2953–2960
Lurz P, von Bardeleben RS, Weber M et al (2021) Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol 77:229–239
Nickenig G, Weber M, Lurz P et al (2019) Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 394:2002–2011
Kodali S, Hahn RT, Eleid MF et al (2021) Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol 77:345–356
Johansson I, Joseph P, Balasubramanian K et al (2021) Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23,000 patients from 40 countries. Circulation 143:2129–2142
Lewis EF, Johnson PA, Johnson W et al (2001) Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 20:1016–1024
Prihadi EA, Delgado V, Leon MB et al (2019) Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. JACC Cardiovasc Imaging 12:491–499
Florescu DR, Muraru D, Florescu C et al (2021) Right heart chambers geometry and function in patients with the atrial and the ventricular phenotypes of functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging 8:211
Badano LP, Muraru D, Enriquez-Sarano M (2013) Assessment of functional tricuspid regurgitation. Eur Heart J 34:1875–1885
Calafiore AM, Lorusso R, Kheirallah H et al (2020) Late tricuspid regurgitation and right ventricular remodeling after tricuspid annuloplasty. J Card Surg 35:1891–1900
Patlolla SH, Schaff HV, Greason KL et al (2021) Early right ventricular reverse remodeling predicts survival after isolated tricuspid valve surgery. Ann Thorac Surg 112:1402–1409
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39
Lancellotti P, Tribouilloy C, Hagendorff A et al (2013) Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 14:611–644
Hahn RT, Zamorano JL (2017) The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging 18:1342–1343
Humbert M, Kovacs G, Hoeper MM, ESC/ERS Scientific Document Group et al (2022) ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 43:3618–3731
Muraru D, Addetia K, Guta AC et al (2021) Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: a three-dimensional echocardiographic study. Eur Heart J Cardiovasc Imaging 22:660–669
Du H, Wonggom P, Tongpeth J et al (2017) Six-minute walk test for assessing physical functional capacity in chronic heart failure. Curr Heart Fail Rep 14:158–166
Navas JP, Martinez-Maldonado M (1993) Pathophysiology of edema in congestive heart failure. Heart Dis Stroke 2:325–329
Cooper G (2018) The impact of chronic oedema on quality of life in the elderly. Br J Community Nurs 23:S10–S12
Seidel HM, Ball JW, Dains JE et al (1995) Heart and blood vessels. In: Schrefer S (ed) Mosby’s Guide to Physical Examination, 3rd edn. Mosby, St. Louis, MO, p 419
Riegel B, Moser DK, Glaser D et al (2002) The Minnesota Living With Heart Failure Questionnaire: sensitivity to differences and responsiveness to intervention intensity in a clinical population. Nurs Res 51:209–218
Brazier JE, Harper R, Jones NM et al (1992) Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 305:160–164
Fam NP, Braun D, von Bardeleben RS et al (2019) Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv 12:2488–2495
Nickenig G, Kowalski M, Hausleiter J et al (2017) Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge MitraClip technique. Circulation 135:1802–1814
Guta AC, Badano LP, Tomaselli M et al (2021) The pathophysiological link between right atrial remodeling and functional tricuspid regurgitation in patients with atrial fibrillation: a three-dimensional echocardiography study. J Am Soc Echocardiogr 34:585–594
Schlotter F, Dietz MF, Stolz L et al (2022) Atrial functional tricuspid regurgitation: novel definition and impact on prognosis. Circ Cardiovasc Interv 15:e011958
Prihadi EA, van der Bijl P, Dietz M et al (2019) Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ Cardiovasc Imaging 12:e008666
Song JM, Jang MK, Kim YJ et al (2010) Right ventricular remodeling determines tricuspid valve geometry and the severity of functional tricuspid regurgitation: a real-time 3-dimensional echocardiography study. Korean Circ J 40:448–453
Haddad F, Couture P, Tousignant C et al (2009) The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg 108:422–33
Kitamura M, Kresoja KP, Balata M et al (2021) Health status after transcatheter tricuspid valve repair in patients with functional tricuspid regurgitation. JACC Cardiovasc Interv 14:2545–2556
Yoshida J, Ikenaga H, Nagaura T et al (2021) Impact of percutaneous edge-to-edge repair in patients with atrial functional mitral regurgitation. Circ J 25(85):1001–1010
Braun D, Rommel KP, Orban M et al (2019) Acute and short-term results of transcatheter edge-to-edge repair for severe tricuspid regurgitation using the MitraClip XTR system. JACC Cardiovasc Interv 12:604–605
Sugiura A, Vogelhuber J, Öztürk C et al (2021) PASCAL versus MitraClip-XTR edge-to-edge device for the treatment of tricuspid regurgitation: a propensity-matched analysis. Clin Res Cardiol 110:451–459
Arnold SV, Chinnakondepalli KM, Spertus JA et al (2019) Health status after transcatheter mitral-valve repair in heart failure and secondary mitral regurgitation: COAPT trial. J Am Coll Cardiol 73:2123–2132
Puls M, Lubos E, Boekstegers P et al (2016) One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J 37:703–712
Taramasso M, Benfari G, van der Bijl P et al (2019) Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol 74:2998–3008
Ledwoch J, Fellner C, Hoppmann P et al (2020) Impact of transcatheter mitral valve repair using MitraClip on right ventricular remodeling. Int J Cardiovasc Imaging 36:811–819
Acknowledgements
This project has been supported by Koeln Fortune Program/Faculty of Medicine, University of Cologne. The graphic abstract has been partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
This manuscript has not been published and is not under consideration for publication elsewhere.
Ethics approval
This study was approved by the local ethics committee of the University of Cologne.
Conflict of interest
Relationship with industry and other entities: C.I. has received travel support by Abbott and consultant honoraria by Abbott and Edwards Lifesciences. R.P. has received speaker and consultant honoraria by Abbott and Edwards Lifesciences. All other authors report no conflict of interest. This project has been supported by Koeln Fortune Program/Faculty of Medicine, University of Cologne.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ehrenfels, MA., Fretter, C., von Stein, J. et al. Role of preexisting right ventricular remodeling in symptoms and prognosis after transcatheter tricuspid valve repair. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02428-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02428-z