Abstract
Background
Growing evidence showing that systemic autoimmune diseases (SADs) are associated with a high risk of atrial fibrillation (AF). However, the impact of SAD on the clinical course of AF patients is largely unknown.
Methods
Retrospective cohort study within a federated healthcare network (TriNetX). Using ICD codes, AF patients on anticoagulant therapy were categorized according to the presence of SAD (M32: Systemic Lupus Erythematosus (SLE); M33: Dermato-polymyositis (DMP); M34: Systemic Sclerosis (SSc); M35: Sjogren syndrome). The primary outcomes were the 5-year risks of (1) all-cause death, (2) thrombotic events (ischemic stroke, acute myocardial infarction, deep vein thrombosis, and pulmonary embolism), and (3) bleeding (intracranial (ICH) and gastrointestinal (GI)). Secondary outcomes were each component of the primary outcomes. Cox regression analysis after propensity score matching (PSM) was used to estimate hazard ratio (HR) and 95% confidence interval (95%CI).
Results
We identified 16,098 AF patients with SAD (68.2 ± 13.4 years; 71.0% female) and 828,772 AF controls (70.7 ± 12.9 years, 41.1% females). After PSM, AF patients with SAD were associated with a higher risk of all-cause death (HR 1.13, 95%CI 1.09–1.71), thrombotic events (HR 1.37, 95%CI 1.32–1.43), and hemorrhagic events (HR 1.41, 95%CI 1.33–1.50) compared to AF controls without SAD. The highest risk of all-cause death and GI bleeding was associated with SSc, while the highest risk of thrombotic events and ICH was associated with SLE.
Conclusion
AF patients with SAD are associated with a high risk of all-cause death, thrombotic, and hemorrhagic events. These patients merit careful follow-up and integrated care management to improve their prognosis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growing evidence shows that patients with systemic autoimmune diseases (SADs) have a high risk of incident atrial fibrillation (AF) [1, 2]. Indeed, the dysregulated inflammatory response that characterizes SAD can contribute to the electrical and structural left atrial remodeling mediated by the inflammasome activation, facilitating the onset and progression of AF [3, 4]. Moreover, SADs are often associated with several cardiovascular risk factors resulting from the multiorgan involvement or even the immunosuppressive treatments [5, 6]. These cardiovascular risk factors are main determinants of both the thromboembolic and hemorrhagic risks in AF patients [7, 8].
Although the number of studies reporting the association between SAD and AF has been rapidly increasing during the last few years, no study has specifically addressed the impact of SAD on the clinical course and outcomes of AF patients on anticoagulant therapy (OAC). The aim of this study was to evaluate the 5-year risk of adverse events in AF-SAD patients compared to AF patients without SAD.
Methods
TriNetX is a research network utilized for several scientific purposes, compliant with the Health Insurance Portability and Accountability Act and the US federal law which protects the privacy and security of healthcare data, including de-identified data as per the de-identification standard of the HIPAA Privacy Rule (https://trinetx.com/real-world-resources/publications/). To gain access to the data in the TriNetX research network, requests are directed to TriNetX and a data sharing agreement is required. As a federated research network, studies using the TriNetX health research network do not need ethical approval as no patient identifiable identification is received.
Study design
This was a retrospective observational study conducted within TriNetX, a global federated health research network with access to electronic medical records (EMRs) from academic and community hospitals covering approximately 80 million individuals, mainly located in the United States. Within this network, available data include demographics; healthcare utilization data (e.g., emergency department, inpatient, and outpatient attendance); diagnoses using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes; laboratory results (Logical Observation Identifiers Names and Codes, LOINC); and medications (RxNorm/Veterans Affairs National Formulary (VANF) codes). More information can be found online (https://trinetx.com/company‐overview/).
Cohort
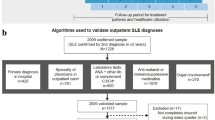
The searches on the TriNetX online research platform were performed on 28 January 2024. Using ICD codes, AF patients (ICD-10-CM I48) on OAC (VANF code: BL110) were categorized into two groups: (1) AF-SAD patients (ICD-10-CM M32: Systemic Lupus Erythematosus (SLE); M33: Dermato-polymyositis (DPM); M34: Systemic Sclerosis (SSc); M35: Sjogren syndrome (SJs)) and (2) AF controls (without: M32–35: SAD or vasculitis, M04: autoinflammatory diseases, and M05–M14: Inflammatory arthropathies). More information about the codes utilized for building each population can be found on Supplementary Table 1. The searches were restricted to a specific time period comprised between 1 January 2000 and 31 December 2018. At the time of the search, 80 participating healthcare organizations had data available for individuals who met the study inclusion criteria. The baseline index event was the AF diagnosis reported in the TriNetX platform. Characteristics registered in the 1 year before the index event were considered the baseline characteristics. The clinical outcomes were identified via ICD-10-CM codes as follows: I63: ischemic stroke, G45: transient cerebral ischemic attack, I75: peripheral arterial thromboembolism, I21: acute myocardial infarction; I82.4: deep vein thrombosis of lower extremity; I26: pulmonary embolism; I60, I61, I62 for intracranial hemorrhage; and K92.1, K92.0, K92.2 for gastrointestinal (GI) bleeding (Supplementary Table 2). All-cause death was recorded using specific variable code within the TriNetX platform.
Outcomes
The primary outcomes were the 5-year risk of (1) all-cause death; (2) a composite thrombotic outcome of ischemic stroke/transient cerebral ischemic attack/peripheral arterial thromboembolism, myocardial infarction, and deep vein thrombosis/pulmonary embolism; and (3) a composite hemorrhagic outcome of intracranial hemorrhage (ICH) and gastrointestinal (GI) bleeding. The secondary outcomes of interest were the 5-year risk of each component of the primary composite outcomes.
We performed a number of some sensitivity analyses to assess the robustness of our primary findings. First, we assessed the risk of primary and secondary outcomes in each SAD compared to AF controls. Second, we assessed the risks of primary outcomes in AF-SAD patients compared to AF controls, considering separately those treated with warfarin and non-vitamin K oral anticoagulants (NOACs). Thereafter, we directly compared AF-SAD patients on warfarin with those on NOAC.
Statistical analyses
Baseline characteristics were compared using chi-squared tests for categorical variables and independent-sample t-tests for continuous variables. Propensity score matching (PSM) 1:1 with neighbor algorithm was used to control the differences in the comparison cohorts. Cohort matching was performed for age at index event, sex, ethnicity, arterial hypertension, diabetes, obesity, dyslipidemia, chronic kidney disease, ischemic heart disease, heart failure, previous cerebral infarction, and cardiovascular medications (β-blockers, antiarrhythmics (class Ia, class Ic, class III), diuretics, statins, antianginals, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and platelet aggregation inhibitors). These variables were chosen because they may influence the risk of primary and secondary outcomes. Absolute standardized mean differences (ASDs) were used to show the distribution of demographic and clinical data among the groups and calculated as the difference in the means or proportions of a particular variable divided by the pooled estimate of standardized differences for that variable. Any baseline characteristic with an ASD < 0.100 was considered well matched. After PSM, Cox regression proportional hazard models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for the risk of primary and secondary outcomes in AF-SAD patients compared to AF controls. Sensitivity analyses were performed as described above.
All tests were two-tailed and p-values of ≤ 0.05 were taken to indicate statistical significance. All analyses were performed in the TriNetX platform which incorporates R (v4.3.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
The initial cohorts consisted of 16,098 AF-SAD patients (68.2 ± 13.4 years, 71.0% females) and 828,772 AF controls without SAD (70.7 ± 12.9 years, 41.1% females). Before PSM, AF-SAD patients were younger, more commonly females, and Black African or Asian, and with a higher prevalence of obesity, hypertension, heart failure, diabetes, dyslipidemia, chronic kidney disease, ischemic heart disease, and previous stroke, compared to AF controls (Table 1).
The number of primary outcomes recorded in AF-SAD patients and AF controls is reported in Table 2. Prior to PSM, AF-SAD patients were associated with a higher risk of all-cause death (HR 1.21, 95%CI 1.18–1.24), thrombotic (HR 1.67, 95%CI 1.63–1.72), and hemorrhagic events (HR 1.77, 95%CI 1.70–1.84) compared to AF controls (Table 2). With regard to the secondary outcomes, AF-SAD patients were at higher risk of stroke, myocardial infarction, deep vein thrombosis, pulmonary embolism, ICH, and GI bleeding compared to AF controls.
After PSM, 15,686 AF patients entered each group and no significative baseline differences were found between the two groups (Table 1). Consistent with the unmatched analysis, AF-SAD patients showed a significant higher risk of primary (Fig. 1, panels A, B, and C; Table 2) and secondary outcomes compared to AF controls (Table 2).
Sensitivity analyses
In the first sensitivity analysis, after PSM, for the comparison with AF controls, we selected for each group (1) 5773 AF-SLE patients, (2) 1625 AF-DMP patients, (3) 1855 AF-SSc patients, and (4) 4694 AF-SJs patients (Supplementary Table 3–6). The number of events for each outcome in each SAD is reported in Table 3. The highest risk of all-cause death was associated with SSc (HR 1.80, 95%CI 1.62–2.03), while the highest risk of composite thrombotic events was associated with SLE (HR 1.44, 95%CI 1.35–1.54). Regarding the risk of composite hemorrhagic events, it was similar in SLE, DMP, and SSc but was the lowest in SJs (Table 3).
Of the secondary outcomes, compared to AF controls, the risk of stroke was significantly higher in SLE, DMP, and SJs yet it was not significant in SSc (Table 3). The risk of myocardial infarction was increased in all SADs except for SSj, whereas the risk of DVT was increased in all AF-SAD patients (Table 3). With regard to hemorrhagic events, SLE was associated with the highest risk of ICH (HR 1.26, 95%CI 1.03–1.53), while the risk of GI bleeding was increased in all SADs (Table 3).
In the second sensitivity analysis, after PSM, we selected for each group 7611 patients on warfarin, 4800 patients on NOAC, and 4733 patients for the direct comparison between AF-SAD patients on warfarin with those on NOAC (Supplementary Table 7–9). In these analyses, the higher risk of primary and secondary outcomes in AF-SAD patients compared to AF controls was consistently independent of OAC type (Table 4). When directly compared, AF-SAD patients on warfarin showed a higher risk of all-cause death, and thrombotic events, and a non-significant trend for a higher risk of bleeding compared to those taking NOACs (Table 4). AF-SAD patients treated with warfarin were associated with a higher risk of deep vein thrombosis compared to those on NOAC, whereas a non-significant trend was found for arterial events, ICH, and GI bleeding (Table 4).
Discussion
In this study, our principal findings are as follows: (1) AF-SAD patients were associated with a higher risk of all-cause death, thrombotic, and hemorrhagic events compared to AF controls; (2) each different SAD is associated with a particular thrombotic and hemorrhagic risk profile; (3) the risk of adverse events in AF-SAD patients was independent of OAC type, although those taking warfarin had a higher risk of mortality and thrombotic events compared to those on NOAC.
In our study, AF-SAD patients had clinical phenotype characterized by younger age, and a high prevalence of female sex, Black African and Asian ethnicity, and cardiovascular risk factors. SAD predilects females and usually arises during the adolescence or young adulthood [9]. The high prevalence of certain ethnicities confirms previous epidemiological studies that have shown that the odds for SAD is higher, and the mean age of disease onset is lower, in Black Africans and Asians compared to Whites [10, 11]. The earlier onset of SAD implies a longer exposure to the inflammatory state that eventually favors an early AF onset with potential anticipation of cardiovascular events.
The higher risk of all-cause death in SAD patients, and particularly in those with SSc, has been previously reported in a retrospective study on 3,150,267 individuals, in which SAD was the leading cause of death among females in England and Wales [12], as well as another retrospective study on 711,247 individuals from the Netherlands, in which SAD was associated with a high mortality rate in females [13].
SAD patients are generally characterized by several risk factors that could increase the risk of death: immunodeficiency may favor the onset of infections or neoplasia; renal involvement can evolve in acute on chronic kidney disease; interstitial lung disease progressing to pulmonary fibrosis may induce respiratory failure, pulmonary hypertension, and heart failure, whereas the prolonged use of steroids can facilitate the onset of secondary Cushing syndrome, diabetes, dyslipidemia, and hypertension [14, 15]. Moreover, the proinflammatory state associated with SAD may heighten the risk of premature atherosclerosis that could lead to an increased risk of arterial events [6], and perturb Virchow’s triad including blood stasis, hypercoagulability, and endothelial injury, leading to an increased risk of venous thromboembolism [16].
Indeed, we found that AF-SAD patients had a higher risk of composite thrombotic outcomes, ischemic stroke, myocardial infarction, and deep venous thrombosis when compared to AF controls. This is in accord with a retrospective study on 98,308 adults with SAD and 198,044 controls enrolled from the MarketScan Commercial Claims databases, where SAD was associated with a sixfold increased risk for venous thromboembolism [17], and with another retrospective study on 136,120 hospitalized patients with SAD from the National Inpatient Sample in the United States, showing that patients with SAD had a higher risk of venous thromboembolism compared to controls [18]. Conversely, in the COMMAND VTE (COntemporary ManageMent AND outcomes in patients with Venous ThromboEmbolism) registry on 2332 patients with acute venous thromboembolism, the high risk for venous thromboembolism in SAD patients was related more to the use of corticosteroids than to the SAD itself [19].
Few studies have analyzed the overall risk of arterial events in SAD patients considering them as a unique clinical entity. A retrospective study on 216,291 hospitalized individuals from the Swedish Hospital Discharge Register showed that patients with SAD had a higher 1-year risk of stroke after discharge (HR 1.50, 95%CI 1.46–1.55) [20], whereas a retrospective study on 79,390 patients hospitalized for myocardial infarction from two Australian population-based datasets showed that SAD was associated with a higher 1-year risk of cardiovascular death (OR 1.71, 95%CI 1.51–1.94) [21].
We found that SLE was associated with the highest risk of composite thrombosis outcome. This has been extensively reported and the reasons are often but not always related to the presence of antiphospholipid antibodies [22]. The development of vasculitis and the enhanced atherosclerosis in SLE could provide other valid explanations [22]. In AF patients with DMP, we found the highest risk of myocardial infarction. In a case–control study on 774 patients with DMP from Canada, the risk of myocardial infarction was increased in DMP patients (HR 6.51, 95%CI 3.15–13.47) [23], whereas in a prospective study on 118 DMP patients followed for 6 years, a 16-fold increased risk of death from myocardial infarction was found [24]. SJs was associated with a high risk of stroke but the data about the risk of cardiovascular events in this disease are controversial. For example, one retrospective study on 4276 SJs patients obtained from the Registry of Catastrophic Illness in Taiwan found that SJs was not associated with a higher risk of stroke (OR 0.84, 95%CI 0.63–1.12), whereas a retrospective study on 102 well-characterized SJs patients showed a significant higher risk of cerebrovascular events (OR 3.83, 95%CI 1.27–11.5) [25].
Finally, we found a 51% higher risk of hemorrhagic events in AF-SAD patients. SSc was associated with the highest risk of GI bleeding, whereas SLE with the highest risk of ICH. This confirms the finding of two retrospective studies from Taiwan that showed a higher incidence of ICH in SLE patients (49.4 vs 10.2 per 100,000 person-year) [26], and a higher risk of GI bleeding in SSc patients (HR 3.93, 95%CI 2.52–6.13) [27]. The higher hemorrhagic risk in SSc was further confirmed by the data of the UK electronic primary care databases that showed a 21% increased risk of any bleeding (HR 1.21, 95%CI 1.00–1.54) [28]. These patients may develop autoantibodies against the coagulation factors VIII and IX leading to the acquired form of hemophilia A or B [29], or develop immune thrombocytopenia as a result of increased turnover or reduced production of platelets [30]. The presence of specific characteristics such as the GI mucosal abnormalities with fibrosis and small vessel vasculopathy in SSc patients or the presence of cerebral aneurysms in SLE patients could further increase this risk.
We noted that both the high thrombotic and the hemorrhagic risk in AF-SAD patients were independent of the OAC type and that patients prescribed NOAC showed a lower risk of all-cause death and thrombosis compared to those on warfarin. Indeed, NOACs are contraindicated in several conditions characterized by a high risk of thrombosis (e.g., antiphospholipid syndrome with triple positivity, advanced liver cirrhosis, end-stage renal disease) in which warfarin is still recommended, and this could have biased the results. Further prospective studies are needed to clarify the best antithrombotic strategies in this high-risk subgroup of AF patients.
The high risks associated with AF-SAD merit a more holistic care approach to managing these patients. Apart from stroke prevention and rhythm management, multidisciplinary cardiovascular preventive strategies, including comorbidity optimization and lifestyle modifications, are needed, aligned with current recommendations in guidelines, for an integrated care approach to AF management [31]. Indeed, adherence to the Atrial Fibrillation Better Care (ABC) pathway is associated with improved clinical outcomes in patients with AF [32, 33].
Limitations
There are several limitations to acknowledge. First, this is a retrospective study and unmeasured bias could have influenced the results. Second, administrative data could fail to identify patients with AF or SAD, affecting the prognosis. Third, although we considered for the PSM the antiarrhythmics therapies, we cannot adjust for ablation or cardioversion procedures that occurred after the index event, for the possibility of introducing “immortal time biases,” thus making it impossible to have a comprehensive overview of those patients treated with rhythm control strategies. Fourth, the outcomes occurring outside the network may have not been well captured and could have influenced the risks associated with the presence or absence of SAD. Fifth, we did not analyze the risk of adverse event in each SAD based on the disease activity or severity, for the lack of these data. Lastly, we did not stratify the analyses based on the age, sex, ethnicity, steroids or immunosuppressive treatments, or the presence of social determinants of health.
Conclusion
AF-SAD patients are associated with a high risk of all-cause death, thrombotic, and hemorrhagic events. These patients merit careful follow-up and integrated care management to improve their prognosis.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Tilly MJ, Geurts S, Zhu F et al (2023) Autoimmune diseases and new-onset atrial fibrillation: a UK Biobank study. Europace 25(3):804–811
Sun G, Ahlehoff O, Fosbol EL et al (2023) Long-term incidence of atrial fibrillation in patients with autoimmune disease. J Am Coll Cardiol 82(20):1969–1971
Ajoolabady A, Nattel S, Lip GYH, Ren J (2022) Inflammasome signaling in atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol 79(23):2349–2366
Gawalko M, Balsam P, Lodzinski P et al (2020) Cardiac arrhythmias in autoimmune diseases. Circ J 84(5):685–694
Conrad N, Verbeke G, Molenberghs G et al (2022) Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 400(10354):733–743
Frostegard J (2005) Atherosclerosis in patients with autoimmune disorders. Arterioscler Thromb Vasc Biol 25(9):1776–1785
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 137(2):263–272
Lip GY (2011) Implications of the CHA(2)DS(2)-VASc and HAS-BLED scores for thromboprophylaxis in atrial fibrillation. Am J Med 124(2):111–114
Voskuhl R (2011) Sex differences in autoimmune diseases. Biol Sex Differ 2(1):1
Lewis MJ, Jawad AS (2017) The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford) 56(suppl_1):i67–i77
Sharma-Oates A, Zemedikun DT, Kumar K et al (2022) Early onset of immune-mediated diseases in minority ethnic groups in the UK. BMC Med 20(1):346
Thomas SL, Griffiths C, Smeeth L, Rooney C, Hall AJ (2010) Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am J Public Health 100(11):2279–2287
Mitratza M, Klijs B, Hak AE, Kardaun J, Kunst AE (2021) Systemic autoimmune disease as a cause of death: mortality burden and comorbidities. Rheumatology (Oxford) 60(3):1321–1330
Albrecht K, Redeker I, Aringer M, Marschall U, Strangfeld A, Callhoff J (2021) Comorbidity and healthcare utilisation in persons with incident systemic lupus erythematosus followed for 3 years after diagnosis: analysis of a claims data cohort. Lupus Sci Med 8(1). https://doi.org/10.1136/lupus-2021-000526
Mason JC, Libby P (2015) Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 36(8):482–489
Borjas-Howard JF, Leeuw K, Rutgers A, Meijer K, Tichelaar V (2019) Risk of recurrent venous thromboembolism in autoimmune diseases: a systematic review of the literature. Semin Thromb Hemost 45(2):141–149
Yusuf HR, Hooper WC, Grosse SD, Parker CS, Boulet SL, Ortel TL (2015) Risk of venous thromboembolism occurrence among adults with selected autoimmune diseases: a study among a U.S. cohort of commercial insurance enrollees. Thromb Res 135(1):50–7
Yusuf HR, Hooper WC, Beckman MG, Zhang QC, Tsai J, Ortel TL (2014) Risk of venous thromboembolism among hospitalizations of adults with selected autoimmune diseases. J Thromb Thrombolysis 38(3):306–313
Yamashita Y, Morimoto T, Kadota K, Ono K, Kimura T (2021) Autoimmune disorders and venous thromboembolism: an update from the COMMAND VTE registry. Eur J Intern Med 84:106–108
Zoller B, Li X, Sundquist J, Sundquist K (2012) Risk of subsequent ischemic and hemorrhagic stroke in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. BMC Neurol 12:41
Van Doornum S, Bohensky M, Tacey MA, Brand CA, Sundararajan V, Wicks IP (2015) Increased 30-day and 1-year mortality rates and lower coronary revascularisation rates following acute myocardial infarction in patients with autoimmune rheumatic disease. Arthritis Res Ther 17(1):38
Bazzan M, Vaccarino A, Marletto F (2015) Systemic lupus erythematosus and thrombosis. Thromb J 13:16
Rai SK, Choi HK, Sayre EC, Avina-Zubieta JA (2016) Risk of myocardial infarction and ischaemic stroke in adults with polymyositis and dermatomyositis: a general population-based study. Rheumatology (Oxford) 55(3):461–469
DeVere R, Bradley WG (1975) Polymyositis: its presentation, morbidity and mortality. Brain 98(4):637–666
Santos CS, Salgueiro RR, Morales CM, Castro CA, Alvarez ED (2023) Risk factors for cardiovascular disease in primary Sjogren’s syndrome (pSS): a 20-year follow-up study. Clin Rheumatol 42(11):3021–3031
Chang YS, Liu CJ, Chen WS et al (2013) Increased risk of subarachnoid hemorrhage in patients with systemic lupus erythematosus: a nationwide population-based study. Arthritis Care Res (Hoboken) 65(4):601–606
Lin YT, Chuang YS, Wang JW, Wu PH (2019) High risk of gastrointestinal hemorrhage in patients with systemic sclerosis. Arthritis Res Ther 21(1):301
Michel A, Gonzalez-Perez A, Saez ME, Garcia Rodriguez LA (2020) Risk of bleeding events among patients with systemic sclerosis and the general population in the UK: a large population-based cohort study. Clin Rheumatol 39(1):19–26
Favaloro EJ, Pasalic L, Lippi G (2022) Autoimmune diseases affecting hemostasis: a narrative review. Int J Mol Sci 23(23). https://doi.org/10.3390/ijms232314715
Ames PRJ, Bucci T, Merashli M, Arcaro A, Gentile F (2023) Thrombocytopaenia in antiphospholipid syndrome: a free radical perspective. Rheumatology (Oxford) 62(6):2070–2075
Chao TF, Joung B, Takahashi Y et al (2022) 2021 Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost 122(1):20–47
Romiti GF, Pastori D, Rivera-Caravaca JM et al (2022) Adherence to the ‘Atrial Fibrillation Better Care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 122(3):406–414
Bucci T, Proietti M, Shantsila A et al (2023) Integrated care for atrial fibrillation using the ABC pathway in the prospective APHRS-AF registry. JACC Asia 3(4):580–591
Author information
Authors and Affiliations
Contributions
TB: writing — original draft, investigation, formal analysis, conceptualization; CC: critically revised the manuscript; MT: supervision, critically revised the manuscript; PRJA: writing — original draft; GYHL: supervision, validation, writing — original draft.
Corresponding author
Ethics declarations
Disclosures
GYHL is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Anthos. No fees are received personally. He is a National Institute for Health and Care Research (NIHR) Senior Investigator and co-principal investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 899871. All other authors report no disclosures.
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bucci, T., Cardamone, C., Triggiani, M. et al. Risk of death, thrombotic and hemorrhagic events in anticoagulated patients with atrial fibrillation and systemic autoimmune diseases: an analysis from a global federated dataset. Clin Res Cardiol 113, 942–950 (2024). https://doi.org/10.1007/s00392-024-02426-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-024-02426-1