Abstract
Background
Accurate risk stratification is important to improve patient selection and outcome of patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR). As epicardial adipose tissue (EAT) is discussed to be involved in cardiovascular disease, it could be useful as a marker of poor prognosis in patients with severe AS undergoing TAVR.
Methods
A total of 416 patients diagnosed with severe AS by transthoracic echocardiography were assigned for TAVR and enrolled for systematic assessment. Patients underwent clinical surveys and 5-year long-term follow-up, with all-cause mortality as the primary endpoint. EAT volume was quantified on pre-TAVR planning CTs. Patients were retrospectively dichotomized at the median of 74 cm3 of EAT into groups with low EAT and high EAT volumes. Mortality rates were compared using Kaplan-Meyer plots and uni- and multivariable cox regression analyses.
Results
A total number of 341 of 416 patients (median age 80.9 years, 45% female) were included in the final analysis. Patients with high EAT volumes had similar short-term outcome (p = 0.794) but significantly worse long-term prognosis (p = 0.023) compared to patients with low EAT volumes. Increased EAT volumes were associated with worse long-term outcome (HR1.59; p = 0.031) independently from concomitant cardiovascular risk factors, general type of AS, and functional echocardiography parameters of AS severity (HR1.69; p = 0.013).
Conclusion
Increased EAT volume is an independent predictor of all-cause mortality in patients with severe AS undergoing TAVR. It can be easily obtained from pre-TAVR planning CTs and may thus qualify as a novel marker to improve prognostication and management of patient with severe AS.
Trial registration
DRKS, DRKS00024479.
Graphical abstract
AS, aortic stenosis; TAVR, transcatheter aortic valve replacement; EAT, epicardial adipose tissue

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe aortic stenosis (AS) is an emerging problem in modern-day aging populations with severe affection of patients’ quality of life and overall survival [1, 2]. Within the past years, transcatheter aortic valve replacement (TAVR) has become an established and safe option for valve replacement [3, 4].
Since TAVR was introduced, numerous parameters including velocity and pressure gradients or calcification were demonstrated to impact on outcome [4,5,6]. This imaging-based risk prediction allows for advanced patient selection and improves procedural safety [7, 8]. Even though parameters for short- and intermediate-term outcome prognostication have been established, [9] data for the prediction of long-term outcome are scarce [10].
With the development of more advanced imaging markers, it is now possible to enhance pathophysiological understanding of different outcomes in TAVR patients [6, 11,12,13].
Epicardial adipose tissue (EAT) is considered a promising marker in cardiovascular disease and current research in cardiology [14]. EAT has paracrine and endocrine function and mediates proinflammatory and oxidative stimuli which nurture cardiovascular disease [15, 16]. As a result, it has been outlined as an important risk factor in various diseases such as coronary artery disease, heart failure, and heart rhythm disorders [17,18,19].
In patients with severe AS undergoing TAVR, EAT was not only associated with short- and medium-term outcome [12] but also with increased rates of post-interventional pacemaker implantation [20], which makes EAT an important factor within this patient group.
However, little is known about the association of EAT and outcome in different subtypes of AS [6] and long-term prognosis in general. As EAT has proven its potential as an outcome predictor, we set out to independently validate previous findings within a well-characterized cohort. Furthermore, we hypothesized that EAT impacts long-term outcome of patients with severe AS undergoing TAVR independently from subtype and other risk factors.
Methods
Patient cohort
A total number of 1395 patients with symptomatic severe AS were scheduled for TAVR between January 2017 and March 2022 at the Heart Center of the University Medical Center of Göttingen. After screening for inclusion and exclusion criteria as previously described [21], a total number of 416 patients were enrolled in this study. All patients were diagnosed according to current guideline recommendations [4] using echocardiography during clinical routine on either a Philips ie33 or a Philips Epic7 (Phillips Healthcare, Eindhoven, Netherlands) system. Images were stored in a picture archiving and communication system and retrospectively reevaluated according to current recommendations by a single observer using Q Station 3.8.5 (Philips Healthcare, Eindhoven, Netherlands) [22]. Patients were assigned to distinct groups of AS (e.g., low-flow low-gradient, high-flow high-gradient) following guideline recommendations [23]. This includes the high-flow high-gradient stenosis with preserved (maximum velocity (Vmax) ≥ 4 m/s, mean pressure gradient (PG) ≥ 40 mmHg, stroke volume index (SVI) > 35 ml/m2, ejection fraction (EF) ≥ 50%; group I) and with reduced ejection fraction (Vmax ≥ 4 m/s, mean PG ≥ 40 mmHg, SVI > 35 ml/m2, EF < 50%; group II) as well as the classical low-flow low-gradient (Vmax < 4 m/s, mean PG < 40 mmHg, SVI < 35 ml/m2, EF < 50%; group III) and paradoxical low-flow low-gradient stenosis (Vmax < 4 m/s, mean PG < 40 mmHg, SVI < 35 ml/m2, EF ≥ 50%; group IV).
Clinical parameters including NYHA class, cardiovascular risk factors, medical history, and laboratory markers were systematically assessed before the TAVR procedure. Furthermore, a record of preexisting heart rhythm disorders, including left bundle branch block; atrial fibrillation; and atrioventricular conduction disorders was documented. The last systematic follow-up assessment was performed in 05/2022 via registry office inquiry. In case of a patient’s death, detailed medical records were obtained from primary care physicians or relatives. The major clinical endpoint was defined as all-cause mortality. Moreover, the presence of a new-onset persistent left bundle branch block, atrio-ventricular block (grade II or higher), and the need of a pacemaker implantation post TAVR were documented. All patients gave written informed consent before participation. The study was approved by the local ethics committee and conducted according to the principles of the Helsinki Declaration.
Computed tomography imaging protocol
Prior to TAVR procedure, patients underwent a standardized imaging protocol for the evaluation of the access path and the device landing zone as recommended by current expert consensus [24]. CT scans were performed on dual-source multi-detector scanners, either a Siemens MAGNETOM Flash CT (Siemens Healthcare, Erlangen, Germany) or a Siemens MAGNETOM Force CT (Siemens Healthcare, Erlangen, Germany) with prospective ECG triggering [25]. Angiography was performed using a high-pitch spiral acquisition mode with bolus tracking in the descending aorta after injection of 80 ml of contrast agent bolus (Imeron 350, Bracco Imaging, Konstanz, Germany) at a flow rate of 4 ml/s, followed by a 40 ml saline chaser with the same flow rate. Scan parameters were set as follows: 2 × 192 × 0.6 mm collimation, 250-ms rotation time, pitch of 3.2, and automated tube current adaption. A dedicated small field of view data set around the heart with medium soft convolution kernel (Siemens Bv36) and a maximum of 1.0-mm slice thickness was reconstructed for further analysis.
Post-processing measurements in CT and calculation of epicardial adipose tissue
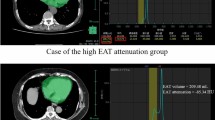
Post processing was performed on a dedicated commercially available software (Syngo.via, Siemens Healthcare, Erlangen, Germany). EAT was outlined by manually contouring the pericardium between the bifurcation of the main pulmonary artery (“cranial border”) and the last visible part of the pericardium (“caudal border”). Afterwards, a three-dimensional volume of interest was computed by interpolation that allowed all further analyses. Using automated thresholds of − 190 to − 30 HU, EAT was visualized (see Fig. 1). Manual corrections were performed if necessary and total volumes of EAT in cubic centimeters were documented. Intra-observer variability was calculated for 50 randomly selected patients. Inter-observer variability was calculated for 20 randomly selected patients, who were remeasured by a second experienced observer who was blinded to the previous results.
Quantification of epicardial adipose tissue using computed tomography. The images show the quantification of epicardial adipose tissue in a basal (left column), midventricular (middle column), and apical (right column) slice position. The top row shows the corresponding cardiac slices. The middle row illustrates the contouring of the pericardium. The bottom row demonstrates the volumetric quantification of all voxels within a range of − 190 to − 30 HU to calculate the epicardial adipose tissue
For calculation of the calcium score, an individual threshold of 550 HU was defined to distinguish between contrast agent and calcifications as recommended [6, 26, 27]. Calcium scoring was performed within the valve leaflets, aortic annulus, or aortic wall up to the sinotubular junction.
TAVR procedure
After evaluation in the interdisciplinary heart-team, TAVR was performed at the University Medical Center Göttingen, which is certified by the German Society of Cardiology.
Most patients received an Edwards Sapien 3™ Valve (61.4%), followed by a Medtronic Evolut™ Pro (25.5%), and Medtronic Evolut™ R (9.8%). A minority of 3.2% received other valves.
Statistical analysis
All statistical analyses were performed using SPSS statistics Version 28.0.0.1 (IBM, Armonk, NY, USA) and GraphPad Prism 9 (GraphPad Software, CA, USA). Normal distribution was tested using the Shapiro–Wilk test. Categorical variables are displayed as frequencies with corresponding percentages and were compared using the chi-square test. Continuous variables are shown as median with corresponding interquartile ranges (IQR) and were compared using the nonparametric Mann–Whitney U test or Kruskal–Wallis test.
Inter- and intra-observer variability was quantified by measuring the bias of two experienced observers as well as by the calculation of the intra-class correlation (ICC). The ICC was reported with the corresponding 95% confidence interval. Results of the ICC were interpreted as suggested by Bobak et al [28]. Furthermore, a coefficient of variation was calculated as \(\mathrm{CoV}\hspace{0.17em}=\hspace{0.17em}\frac{\mathrm{standard}\;\mathrm{deviation}\;\mathrm{of}\;\mathrm{differences}}{\mathrm{mean}\;\mathrm{of}\;\mathrm{differences}}\).
After dichotomization at the median, survival analyses were performed and illustrated by Kaplan–Meier plots. Mortality was compared using the log-rank test. Proportional hazard assumption was verified by statistically testing the variables’ interaction with time by comparing the time-dependent covariable with the original covariable. To obtain hazard ratios (HR) of individual parameters, a logistic univariable cox regression analysis was performed. Parameters with a significant association (p < 0.05) with the primary endpoint were included for further analysis in multivariable cox regression analyses after adjustment for age, sex, and BMI. To avoid overfitting, two different models were tested, a first one including cardiovascular risk factors and the type of aortic stenosis, and a second one including specific parameters characterizing the subtypes of aortic stenosis. Significance levels below 0.05 are considered as statistically significant.
Results
Patient cohort
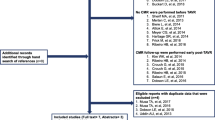
From the initially recruited 416 patients, 29 had to be excluded due to rescheduling for surgical or trans-apical valve replacement. Thirty-four patients had to be excluded as they were retrospectively reclassified as moderate AS before the procedure. Four patients withdrew their consent for participation before the procedure. One patient died before intervention due to infective endocarditis of the aortic valve. In three patients, the caudal end of the heart was not included within the cardiac acquisition, and 4 patients had their cardiac CT performed at external centers prior to intervention and had to be excluded from further analysis. After retrospective dichotomization at the median of the total EAT volume in the cohort, 171 patients were allocated to the low EAT volume group (≤ 74 cm3) and 170 patients were assigned to the high EAT volume group (> 74 cm3). An overview is displayed in Fig. 2.
Reproducibility
EAT volumes as calculated by CT had excellent reproducibility with an ICC of 0.978 (0.960–0.987; p < 0.001) and a CoV of 9.3%. Inter-observer variability was comparably good with an ICC of 0.994 (0.984–0.997; p < 0.001) and a CoV of 5.2%.
Baseline characteristics
The median age of the cohort was 80.9 (76.3–83.9) years with no differences between the high and the low EAT volume group. One hundred fifty-two (45%) patients were female, with more female patients in the low EAT volume group compared to the high EAT volume group (89 vs. 63; p = 0.005). Patients in the high EAT volume group had a higher BMI (28.8 vs. 25.0; p < 0.001), higher NYHA classes (p = 0.016), and a higher prevalence of diabetes mellitus type II (47 vs. 73; p = 0.003). Other comorbidities and cardiovascular risk factors were equally distributed between both groups (compare Table 1).
The baseline characteristics and echocardiographic indices of patients with different types of AS are summarized in Table 2. For the EAT volume, no differences between the individual groups could be found (I 75.0 vs. II 86.3 vs. III 71.1 vs. IV 68.3 cm3; p = 0.822).
Echocardiography
Patients in the high and the low EAT volume groups had no differences between ejection fraction, mean PG, or maximum flow velocity over the aortic valve (see Fig. 3). In the group of patients with low EAT volumes, the aortic valve area was slightly smaller compared to the group of patients with high EAT volumes (0.65 vs. 0.70, 0.011) (see Table 1).
Characteristics of the aortic stenosis in patients with high and low EAT volumes. The image shows the median (interquartile range) of the ejection fraction, the maximum flow velocity, and mean pressure gradient of the aortic valve in patients with low (≤ 75 cm3) and high (> 75 cm3) epicardial adipose tissue (EAT)
Outcome and EAT
There were no differences between short-term outcome within 30 days after TAVR (log rank p = 0.591) (see Fig. 4A). In contrast, patients with higher EAT volumes had a significantly higher 5-year all-cause mortality compared to patients with low EAT volumes (log rank p = 0.023) (see Fig. 4B). As shown in Table 3, there were no differences regarding the occurrence of heart rhythm disorders after TAVR between the high and low EAT volume group. There was only a statistical trend towards a higher rate of pacemaker implantations post-TAVR in patients with higher EAT volume (15 vs. 25, p = 0.088).
Survival after TAVR. The top graph (A) shows the 30-day survival after TAVR in the low (red) and high (blue) EAT volume group. The bottom graph (B) displays the long-term 5-year survival after TAVR in the low (red) and high (blue) EAT volume group. A p-value below 0.05 was considered statistically significant. EAT, epicardial adipose tissue; TAVR, transfemoral aortic valve replacement
In univariable cox regression analysis, presence of diabetes mellitus (HR 1.63, p = 0.007), a positive family history of cardiovascular events (HR 0.48; p = 0.048), peripheral artery disease (HR 2.0; p = 0.003), and the type of AS (HR 1.37; p < 0.001) were associated with increased mortality after TAVR (see Table 4).
Furthermore, EAT volumes above the median predicted a poorer 5-year long-term outcome with a HR of 1.48 (1.04–2.12; p = 0.032). In multivariable cox regression analysis, after adjustment for age, sex, and BMI, increased EAT volumes above the median still showed a significant association with mortality after TAVR (HR 1.59 1.04–2.41; p = 0.031) independently from cardiovascular risk factors, comorbidities, and AS subtype (see Table 4). In addition, increased EAT volume above the median was the only parameter remaining associated with increased mortality after TAVR in multivariable cox regression analysis including specific parameters characterizing the aortic stenosis like the SVI, Vmax, mean PG, or the calcium scoring (HR 1.69 1.12–2.54; p = 0.013) (Table 5).
Discussion
Risk stratification in patients undergoing TAVR is important to enhance the outcome of the procedure and to improve selection of patients that would benefit from interventional therapies [29, 30]. In addition to established parameters, calculation of EAT volume using cardiac CT offers a valuable tool for improved risk stratification. While the short-term outcome did not differ between patients with high and low EAT volumes, increased EAT volumes predicted a worse long-term outcome in TAVR patients. This was independent of patients’ cardiovascular risk factors as well as the subgroup of severe AS. Interestingly, EAT volumes had no impact on cardiovascular conduction disorders after TAVR, although we observed a trend towards a higher rate of pacemaker implantations in patients with high EAT volumes.
As EAT volumes can be sufficiently assessed from pre-TAVR planning CTs, its quantification can be performed without the need for additional diagnostic procedures or the application of additional radiation.
Conventional cardiovascular risk factors were validated for risk stratification in patients with severe AS, and summarized in scores such as the EUROSCORE II to assign patients for SAVR or TAVR [31]. However, it was shown that those scores require adjustments within the population of patients scheduled for TAVR to improve outcome prediction [32,33,34].
An emerging parameter receiving significant attention in cardiovascular medicine is EAT providing novel insights into pathophysiology in various cardiovascular diseases [14, 35, 36].
EAT was shown to be a risk factor in atherosclerotic coronary artery disease and is independently associated with a higher risk of obstructive CAD, atrial fibrillation, and heart failure. [18, 19, 36,37,38] Moreover, increased EAT volumes are associated with increased rates of cardiovascular events and worse outcome independent of conventional cardiovascular risk factors even in the general population [17].
Pathophysiologically, the interconnection of EAT and cardiovascular remains a matter of debate. On the one hand, EAT is assumed to generate proinflammatory cytokines causing structural damage and promoting adverse remodeling of adjacent tissue within the myocardium and attached structures [14, 16]. Moreover, as EAT is significantly associated with increased coronary artery calcification, it is also suspected to promote atherosclerosis [39, 40]. Likewise, it could be an important inflammatory mediator contributing to aortic valve calcification in the same way [41]. In the present study, this was partly confirmed by higher aortic calcium scores in the group of patients with increased EAT volumes. However, this direct involvement of EAT in the development and progression of AS has yet to be demonstrated.
On the other hand, the causal interaction of EAT with cardiovascular diseases could rather be bidirectional. Cardiovascular diseases and risk factors like atherosclerosis, atrial fibrillation, diabetes, or obesity may cause systemic dysregulation in the first place which is then promoting the development, progression, and remodeling of EAT as a symptom-like phenomenon. This is partly paralleled by the observation of a protective role of EAT in early years of life and a consecutive remodeling of EAT at later age stages causing adverse effects on the cardiovascular system [14, 42]. The complex interaction of cardiovascular disease and EAT mutually promoting each other and leading to the observed association of EAT with pathological cardiovascular conditions will need to be further investigated in prospective studies including dedicated control groups.
Therefore, increased volumes of EAT in patients might just represent a generally sicker group of patients in more advanced stages of their disease, which is concordantly indicated by higher NYHA classes in the group of patients with higher EAT volumes.
Since EAT volumes can be easily quantified on conventional TAVR planning CTs (that are being performed in any given patient) without the need for an additional diagnostic procedure or radiation exposure, it may well be of predictive value in this patient population.
Interestingly, total CT-derived EAT volumes are very variable across different study populations ranging from a median of 71 to 90 ml in younger patient populations with cardiovascular disease [43,44,45,46] up to 127 to 130 ml in older patient populations [12, 20]. EAT volumes of the present study are rather low, despite a similar age and risk factor distribution of the current cohort compared to patients in the latter studies. While this could also be attributed to distinct patient characteristics, it is more likely that methodological differences in the evaluation of EAT volumes are accountable for the heterogeneity.
While the cranial and caudal border for EAT volume quantification was mostly the same in all studies, a large meta-analysis already reported that different Hounsfield ranges were used for EAT volume evaluation as well as some studies analyzed EAT volumes in more systolic or diastolic cardiac phases [47]. Other factors like image resolution, the method of reconstruction, and the analysis of contrast-enhanced or non-contrast scans could further influence EAT measurements. This emphasizes the need for further standardization with regard to measurement techniques before utilizing EAT volumes for risk stratification in clinical routine.
Despite potential methodological divergences, the assessment of EAT volumes was reported to be a valuable prognostic tool in cardiovascular disease in general [17, 37, 47,48,49]. Only a few studies investigated the impact of EAT volumes on the outcome of patients with AS undergoing TAVR. Even though total EAT volumes are different to previously investigated populations, first studies have already outlined that EAT quantification can enhance risk prediction in TAVR patients [50] and higher EAT volumes are associated with worse short-term and intermediate-term outcome [12]. While this study was now able to add implications on long-term outcome over a 5-year period, we were not able to reproduce earlier findings on the short-term outcome. On the one hand, this may be attributed to the low number of events within 30 days after TAVR. On the other hand, it could be a consequence of differing EAT volumes, patient comorbidities, or procedural differences. For example, another study reported significant associations between relevant heart rhythm disorders and higher amounts of EAT [20], which is a well-known short-term complication after TAVR. In contrast, the present study revealed similar rates of post-TAVR conduction abnormalities between both groups potentially indicating different patient selection or intra- and post-procedural management.
Furthermore, it should be noted that earlier trials reported a paradoxical effect of obesity in patients undergoing TAVR as obese patients have been observed to have a lower mid- and long-term mortality compared to patients having normal weight [51,52,53]. However, in the present study, patients with increased EAT volume had a concordantly increased BMI but worse outcome. While the association of increased EAT volumes and obesity was described earlier [54], the worse outcome of those patients is contradictive to the protective effect of obesity. Interestingly, in the present study, patients with both high and low EAT volumes belong to the same subgroup of overweight but non-obese patients with a BMI between 25 and 29.9 kg/m2. Patients in this BMI range were defined as a single population in the abovementioned studies and compared with patients in other BMI ranges. This could partly explain the smaller impact of the BMI on outcome in this study. Furthermore, as patients belonged to the same BMI range, the reported difference in mortality potentially points out to an additional value of EAT for sub-characterization in overweight patients.
While the inhomogeneity of the disease alone with different types of severe AS (e.g., low-flow low-gradient vs. high-flow high-gradient) has implications on outcome [55], this study could now add that EAT volume quantification allows an independent risk stratification in TAVR patients independent of the subgroup of severe AS. Therefore, the addition of EAT volume as an outcome predictor in TAVR patients could add a valuable, independent outcome predictor in distinct types of severe AS [56]. Still, the disparate findings on short-term outcome and conduction abnormalities in other studies endorse the need for multiparametric outcome prediction in TAVR patients and a validation in larger prospective trials.
Using EAT volume as a predictive parameter for long-term outcome assessments is especially interesting considering that TAVR is progressively used in a younger patient collective with a longer life expectancy [57]. Multiparametric CT including prognostication with EAT volumes may therefore have important implications on interventional decision-making beyond valve size and prosthesis selection.
Limitations
Findings of this study are based on data from a single-center cohort, within a certified and specialized institution for TAVR. Mortality rates and causes might differ in multicentric studies. The rather small number of patients within this study potentially reduces the statistical accuracy and implies that some results may or may not be relevant in larger cohorts.
The calculation of EAT volumes using CT Hounsfield units is just an estimation of the true epicardial fat volume and might also include other parts of surrounding tissue especially pericardial fat tissue. The threshold of 75 cm3 of EAT volume might only apply to the investigated cohort and not be applicable in other patient groups. Further studies in prospective, population-based investigations are therefore required. Without a direct comparison with a matched control group, the impact of EAT might just be a surrogate for the influence of other comorbidities or life-style factors. Despite multivariable regression analyses, the influence of other given pathologies on outcome cannot be fully excluded and implications of EAT in TAVR patients must be further investigated.
Conclusion
Patients with severe AS and higher volumes of EAT have worse long-term but not short-term outcome after TAVR. Increased amounts of EAT predict long-term mortality and potential necessity for secondary prevention independently from the type of aortic valve stenosis, its treatment with TAVR, or accompanying cardiovascular risk factors.
As EAT volumes can be easily quantified using regular TAVR planning CTs without the need for additional diagnostic tests or radiation exposure, it could qualify as an additional prognostic and cost-effective marker in TAVR patients. Its calculation could improve risk stratification and care of patients with severe AS assigned for TAVR.
References
Martinsson A et al (2015) Temporal trends in the incidence and prognosis of aortic stenosis. Circulation 131:988–994
Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K (2013) The evolving epidemiology of valvular aortic stenosis. The Tromsø Study Heart 99:396–400
Tsigkas G et al (2018) Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis. J Geriatr Cardiol 15:76–85
Vahanian A et al (2022) ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 43:561–632
Snir AD et al (2021) Prevalence and outcomes of low-gradient severe aortic stenosis—from the National Echo Database of Australia. J Am Heart Assoc 10:e021126
Ludwig S et al (2020) TAVR for low-flow, low-gradient aortic stenosis: prognostic impact of aortic valve calcification. Am Heart J 225:138–148
Wolff G et al (2021) Risk modeling in transcatheter aortic valve replacement remains unsolved: an external validation study in 2946 German patients. Clin Res Cardiol 110:368–376
Edwards FH et al (2016) Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiology 1:46–52
Yamamoto M et al (2021) Clinical risk model for predicting 1-year mortality after transcatheter aortic valve replacement. Catheter Cardiovasc Interv 97:E544–E551
Duncan A et al (2015) Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv 8:645–653
Puls M et al (2020) Impact of myocardial fibrosis on left ventricular remodelling, recovery, and outcome after transcatheter aortic valve implantation in different haemodynamic subtypes of severe aortic stenosis. Eur Heart J 41:1903–1914
Eberhard M et al (2019) Epicardial adipose tissue volume is associated with adverse outcomes after transcatheter aortic valve replacement. Int J Cardiol 286:29–35
Evertz R et al (2023) Aortic valve calcification and myocardial fibrosis determine outcome following transcatheter aortic valve replacement. ESC Heart Failure 10:2307–2318
Iacobellis G (2022) Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol 19:593–606
Oikonomou EK, Antoniades C (2019) The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 16:83–99
van Woerden G et al (2022) Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: mechanisms, management and modern perspectives. Eur J Heart Fail 24:2238–2250
Mahabadi AA et al (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall study. J Am Coll Cardiol 61:1388–1395
van Woerden G et al (2021) Importance of epicardial adipose tissue localization using cardiac magnetic resonance imaging in patients with heart failure with mid-range and preserved ejection fraction. Clin Cardiol 44:987–993
Zhou M, Wang H, Chen J, Zhao L (2020) Epicardial adipose tissue and atrial fibrillation: possible mechanisms, potential therapies, and future directions. Pacing Clin Electrophysiol 43:133–145
Weferling M et al (2021) Epicardial fat volume is associated with preexisting atrioventricular conduction abnormalities and increased pacemaker implantation rate in patients undergoing transcatheter aortic valve implantation. Int J Cardiovasc Imaging. https://doi.org/10.1007/s10554-021-02502-x
Beuthner BEC et al (2020) Interdisciplinary Research on Aortic Valve Stenosis: a longitudinal collection of biospecimens and clinical data of patients undergoing transcatheter aortic valve replacement. Open J Bioresour 7(1):3. https://doi.org/10.5334/ojb.65
Lang RM et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1-39.e14
Baumgartner Chair H et al (2017) Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 18:254–275
Blanke P et al (2019) Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 13:1–20
Evertz R et al (2022) Impact of observer experience on multi-detector computed tomography aortic valve morphology assessment and valve size selection for transcatheter aortic valve replacement. Sci Rep 12:21430
Evertz R et al (2021) Head-to-head comparison of different software solutions for AVC quantification using contrast-enhanced MDCT. J Clin Med 10:3970
Seiffert M et al (2016) Device landing zone calcification and its impact on residual regurgitation after transcatheter aortic valve implantation with different devices. Eur Heart J Cardiovasc Imaging 17:576–584
Bobak CA, Barr PJ, O’Malley AJ (2018) Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med Res Methodol 18:93
Cocchia R et al (2017) Patient selection for transcatheter aortic valve replacement: a combined clinical and multimodality imaging approach. World J Cardiol 9:212–229
Mylotte D, Martucci G, Piazza N (2012) Patient selection for transcatheter aortic valve implantation: an interventional cardiology perspective. Ann Cardiothorac Surg 1:206–215
Nashef SAM et al (2012) EuroSCORE II. Eur J Cardiothorac Surg 41:734–744;discussion 744–745
Baro R, Cura F, Belardi J, Brugaletta S, Lamelas P (2021) Surgical risk scoring in TAVR: still needed? A metaregression analysis. Curr Probl Cardiol 46:100875
Khan AA, Murtaza G, Khalid MF, Khattak F (2019) Risk stratification for transcatheter aortic valve replacement. Cardiol Res 10:323–330
Strom JB et al (2021) Role of frailty in identifying benefit from transcatheter versus surgical aortic valve replacement. Circ Cardiovasc Qual Outcomes 14:e008566
Brandt V et al (2022) Additive value of epicardial adipose tissue quantification to coronary CT angiography-derived plaque characterization and CT fractional flow reserve for the prediction of lesion-specific ischemia. Eur Radiol 32:4243–4252
Jin X et al (2022) Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail 24:1346–1356
Braescu L et al (2022) The role and implications of epicardial fat in coronary atherosclerotic disease. J Clin Med 11:4718
Yu W et al (2021) Association of epicardial fat volume with increased risk of obstructive coronary artery disease in Chinese patients with suspected coronary artery disease. J Am Heart Assoc 10:e018080
Mahabadi AA et al (2014) Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging 7:909–916
Cosson E et al (2021) Epicardial adipose tissue volume and coronary calcification among people living with diabetes: a cross-sectional study. Cardiovasc Diabetol 20:35
Parisi V et al (2015) Epicardial adipose tissue has an increased thickness and is a source of inflammatory mediators in patients with calcific aortic stenosis. Int J Cardiol 186:167–169
Konwerski M, Gąsecka A, Opolski G, Grabowski M, Mazurek T (2022) Role of epicardial adipose tissue in cardiovascular diseases: a review. Biology (Basel) 11:355
Alexopoulos N et al (2010) Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 210:150–154
Djaberi R et al (2008) Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol 102:1602–1607
Cheng VY et al (2010) Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging 3:352–360
Bettencourt N et al (2012) Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol 158:26–32
Chong B et al (2023) Epicardial adipose tissue assessed by computed tomography and echocardiography are associated with adverse cardiovascular outcomes: a systematic review and meta-analysis. Circ Cardiovasc Imaging 16:e015159
Mancio J et al (2018) Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 19:490–497
Kunita E et al (2014) Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis 233:447–453
Davin L et al (2019) Epicardial adipose tissue and myocardial fibrosis in aortic stenosis relationship with symptoms and outcomes. JACC Cardiovasc Imaging 12:213–214
Seo J et al (2022) A meta-analysis on the impact of high BMI in patients undergoing transcatheter aortic valve replacement. Journal of Cardiovascular Development and Disease 9:386
Sharma A et al (2020) Relationship of body mass index with outcomes after transcatheter aortic valve replacement: results from the National Cardiovascular Data-STS/ACC TVT Registry. Mayo Clin Proc 95:57–68
McInerney A et al (2021) Impact of morbid obesity and obesity phenotype on outcomes after transcatheter aortic valve replacement. J Am Heart Assoc 10:e019051
Rabkin SW (2007) Epicardial fat: properties, function and relationship to obesity. Obes Rev 8:253–261
Maréchaux S et al (2019) Prognostic value of low flow in patients with high transvalvular gradient severe aortic stenosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging 12:e009299
Golbus JR, Bach DS (2019) Severe aortic stenosis. Circulation. Cardiovascular Imaging 12:e009834
Hanzel GS, Gersh BJ (2020) Transcatheter aortic valve replacement in low-risk, young patients. Circulation 142:1317–1319
Funding
Open Access funding enabled and organized by Projekt DEAL. German Research Foundation (DFG, CRC 1002, D1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schulz, A., Beuthner, B.E., Böttiger, Z.M. et al. Epicardial adipose tissue as an independent predictor of long-term outcome in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02387-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02387-5