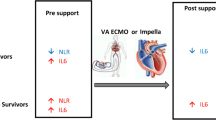
Abstract
Background
Mortality in cardiogenic shock (CS) remains high even when mechanical circulatory support (MCS) restores adequate circulation. To detect a potential contribution of systemic inflammation to shock severity, this study determined associations between C-reactive protein (CRP) concentrations and outcomes in patients with CS.
Methods
Unselected, consecutive patients with CS and CRP measurements treated at a single large cardiovascular center between 2009 and 2019 were analyzed. Adjusted regression models were fitted to evaluate the association of CRP with shock severity, 30-day in-hospital mortality and treatment response to MCS.
Results
The analysis included 1116 patients [median age: 70 (IQR 58–79) years, 795 (71.3%) male, lactate 4.6 (IQR 2.2–9.5) mmol/l, CRP 17 (IQR 5–71) mg/l]. The cause of CS was acute myocardial infarction in 530 (48%) patients, 648 (58%) patients presented with cardiac arrest. Plasma CRP concentrations were equally distributed across shock severities (SCAI stage B–E). Higher CRP concentrations were associated with 30-day in-hospital mortality (8% relative risk increase per 50 mg/l increase in CRP, range 3–13%; p < 0.001), even after adjustment for CS severity and other potential confounders. Higher CRP concentrations were only associated with higher mortality in patients not treated with MCS [hazard ratio (HR) for CRP > median 1.50; 95%-CI 1.21–1.86; p < 0.001], but not in those treated with MCS (HR for CRP > median 0.92; 95%-CI 0.67–1.26; p = 0.59; p-interaction = 0.01).
Conclusion
Elevated CRP concentrations are associated with increased 30-day in-hospital mortality in unselected patients with cardiogenic shock. The use of mechanical circulatory support attenuates this association.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiogenic shock (CS) remains a clinical challenge with a high mortality despite significant advances in overall cardiovascular medicine [1, 2]. The high mortality of CS likely reflects its complex and incompletely understood pathophysiology involving hemodynamic and circulatory disturbances, triggering systemic inflammation and organ dysfunction [3]. The degree of CS severity can be graded using the Society for Cardiovascular Angiography and Intervention (SCAI) shock classification and is associated with mortality in patients with CS [4, 5].
Only early percutaneous coronary intervention (PCI) of the infarct-related artery has been shown to improve survival in patients with CS caused by acute myocardial infarction (AMI), but the associated mortality risk remains high [6, 7]. Current hopes have been set in mechanical circulatory support devices (MCS) as a bridge to recovery, restoring cardiac output and tissue perfusion; yet the evidence on this is far from definitive and they come at its own cost of more complications [8,9,10,11].
Non-cardiovascular factors influence both, prognosis and response to treatment in patients with CS [12,13,14]. Systemic inflammation, as indicated by elevated plasma concentrations of inflammatory mediators such as C-reactive protein (CRP), is frequently observed among patients with CS and considered a major pathophysiologic mechanism contributing to worsening shock and multi-organ injury [3, 15,16,17,18,19]. In patients with sepsis induced CS treatment with MCS might even be associated with a higher survival as indicated by a retrospective analysis [20]. We, therefore, hypothesized that systemic inflammation may be a key factor contributing to the downward spiral of CS characterized by aggravated tissue hypoperfusion. And in turn, this patient group in particular may benefit from MCS restoring blood flow and providing improved tissue perfusion.
Hence, this study is aimed to explore the association of systemic inflammation with shock severity, 30-day in-hospital mortality, and therapy response in patients with CS. Improving its understanding might facilitate the development of successful treatment strategies and conduction of clinical trials in this field.
Methods
Study design
This is a retrospective analysis in the previously reported [4] monocentric Hamburg Cardiogenic Shock registry of the University Heart and Vascular Center Hamburg. The registry includes patients aged 18 years or older suffering from CS between 2009 and 2019. To identify eligible patients, patient records were electronically scanned for patients with CS according to ICD-10 coding (R.57). All records were manually validated by a physician to confirm CS as the primary cause of the shock leading to hospital admission. Patients with a primarily septic shock were not included. This was verified by a manual chart review (documented clinical and laboratory data) of all included cases. Baseline data, comorbidities, and treatment data were collected in a dedicated database. Patients were followed up until death or discharge from hospital.
The aim of this study was to explore the association of systemic inflammation with shock severity, 30-day in-hospital mortality, and therapy response in patients with CS. As surrogate parameter for systemic inflammation plasma CRP concentrations on admission were used, so that patients without available data for plasma CRP concentrations were excluded from this study. The severity of CS was defined based on the published SCAI shock classification [21] as well as on the use and number of vasopressors. Also, as surrogate for shock duration lactate measurements on admission were evaluated in relation to plasma CRP concentrations. Therapy response was assessed as survival with use of MCS (Veno-arterial extracorporeal membrane oxygenation therapy (VA-ECMO) and/or Impella®).
This study conforms with the principles outlined in the Declarations of Helsinki, and was approved by our local ethics committee (registration code PV5607). Due to the retrospective study design all patient data were anonymized, hence the need to give informed consent was waived.
Statistical analyses
Baseline characteristics were generated for the whole cohort, as well as for the subgroups with high vs. low plasma CRP concentrations, dichotomized by CRP median of the study cohort. Continuous variables are expressed as medians with interquartile range and compared using the Mann–Whitney U test. Categorical variables are shown as absolute numbers and percentages and compared using the chi2 test. p-values < 0.05 were considered significant.
To evaluate the association between plasma CRP concentrations and SCAI stage linear and logistic regression models were fitted, using SCAI B as reference category. Plasma CRP concentrations were first analyzed as continuous variable per 50 mg/l CRP increase (linear regression) and subsequently as dichotomous variable using the CRP median (logistic regression). To examine the relationship between plasma CRP concentrations and lactate concentrations (as a surrogate for CS duration) as well as the use and number of vasopressors linear and logistic regression models were chosen correspondingly.
To assess the relative risk of 30-day in-hospital mortality in association with plasma CRP concentrations, Kaplan–Meier method was applied and Cox regression models were fitted, again using plasma CRP as a continuous and/or dichotomous variable as stated above. Groups were compared using the log-rank test. To evaluate the relative risk of 30-day in-hospital mortality in association with plasma CRP concentrations within each SCAI stage subgroup analyses including only patients with SCAI stage B and C combined, D and E were performed.
To evaluate the interaction between plasma CRP concentrations and MCS use regarding the relative risk of 30-day in-hospital mortality an interaction term between plasma CRP concentrations and MCS use was fitted into the above-described cox regression models. Analyses were performed for MCS use in general and each device separately. To account for differences in patient, presentation and shock-specific characteristics in patients with high vs. low plasma CRP concentrations, all analyses were adjusted for the following potential confounders based on clinical knowledge and prior studies [22]: age, sex, AMI, lactate, pH, cardiopulmonary resuscitation (CPR), patient intubated due to index event and SCAI stage (if not the endpoint). For the multivariable adjusted regression models, only patients with data on all confounders were considered (e.g., complete case analysis).
All analyses were performed using the software R version 4.0.3.
Results
Baseline characteristics
The data set contains information from 1338 patients with CS unselected for its etiology. 201 patients without information about plasma CRP concentrations were excluded, so that 1116 patients remained for this analysis. Baseline characteristics of the overall cohort and of those with high and low CRP concentrations (split by median CRP) are displayed in Table 1.
Median age of all patients was 70 (58–78.6) years and 795 (71.3%) were male. Almost half of the patients presented with CS due to AMI (n = 530, 47.7%) and prior cardiac arrest (CA) (n = 648, 58.3%). Median pH was 7.3 (7.1–7.4), lactate 4.6 (2.2–9.5) mmol/l and GFR 41.6 (26.9–57.9) ml/min. The overall distribution of SCAI stages was B: 45 patients (4.1%), C: 569 patients (52%), D: 254 patients (23.2%) and E: 227 patients (20.7%). MCS was used in 314 patients (28.2%), including VA-ECMO in 257 patients (23.1%) and Impella® in 158 patients (14.2%). 101 patients (9.1%) were treated with both VA-ECMO and Impella®. The median plasma CRP concentration in the study population was 17 (5.0–71.0) mg/l. Patients with CRP > median were slightly older (CRP > median: 71 (61–79) years; CRP ≤ median: 68 (55–78) years) and less often male (CRP > median: n = 376, 68.7%; CRP ≤ median: n = 419, 73.8%). The prevalence of CS due to AMI and CA was slightly reduced in the group of patients with CRP > median (AMI: CRP > median: n = 240, 44.0%; CRP ≤ median: n = 290, 51.1% and CA: CRP > median: n = 265, 48.7%; CRP ≤ median: n = 383, 67.4%). The use of MCS was slightly less observed in patients with CRP > median (CRP > median: MCS: n = 134, 24.6% with VA-ECMO: n = 106, 19.5% and Impella®: n = 69, 12.7%; CRP ≤ median: MCS: n = 180, 31.6% with VA-ECMO: n = 151, 26.5% and Impella®: n = 89, 15.6%).
Systemic inflammation and shock severity
As the SCAI shock stage (shock severity) increased, plasma CRP concentrations appeared to decrease but after adjustment for relevant confounders, there was no consistent association between plasma CRP concentrations and shock severity, irrespective of being considered as continuous [beta (β) per 50 ml/l CRP increase with reference SCAI B: SCAI C (β − 0.11, 95% confidence interval (CI): − 0.61, 0.38; p = 0.66), SCAI D (β − 0.03, 95%-CI − 0.54, 0.48; p = 0.91); SCAI E (β − 0.28, 95%-CI − 0.83, 0.27; p = 0.32)] or dichotomized variable [Odds Ratio (OR) for CRP > median: SCAI C (OR 0.53, 95%-CI 0.26, 1.03; p = 0.066), SCAI D (OR 0.83, 95%-CI 0.41, 1.65; p = 0.6); SCAI E (OR 0.44, 95%-CI 0.21, 0.92; p = 0.032)] as shown in Fig. 1.
Association between plasma CRP concentrations and shock severity (SCAI stage) in patients with cardiogenic shock. A Percental distribution of CRP > vs. ≤ median per SCAI stage. B Logistic regression with odds ratio (OR) for CRP > median. C Linear regression per 50 mg/l CRP increase. CRP: C-reactive protein. SCAI-stage: shock stage classification by the Society for Cardiovascular Angiography and Interventions. 95%-CI 95% confidence interval. All regression models adjusted for age, sex, acute myocardial infarction, lactate, pH, cardiopulmonary resuscitation (CPR) and patient intubated due to index event
Higher CRP concentrations were associated with higher lactate concentrations (i.e., presumably a longer duration of CS). When considered as dichotomized variable this association persisted even after adjustment for relevant confounders including SCAI stage (OR for CRP > median: 1.06, 95%-CI 1.02, 1.10; p = 0.003). When considered as continuous variable this association was only statistically significant before but not after adjustment (β per 50 ml/l CRP increase: 0.01, 95%-CI − 0.02, 0.04; p = 0.47).
Higher CRP concentrations were partly associated with use of vasopressors. Detailed results are given in Appendix Table 2.
Systemic inflammation and 30-day mortality
Patients were followed-up for a median in-hospital follow-up time of 25 (95%-CI 23, 28) days. A total of 626 patients died within the 30-day follow up, yielding a 30-day in-hospital mortality rate of 61.47% (95%-CI 58.09, 64.58%). Patients with higher CRP concentrations had a higher 30-day in-hospital mortality [62.68% (95%-CI 57.97, 66.86%)] than patients with lower CRP concentrations [60.49% (95%-CI 55.41, 65.0%)] (Fig. 2). Kaplan–Meier survival curves are displayed in Appendix Fig. 4.
After adjustment for potential confounders, higher plasma CRP concentrations were associated with a higher risk of 30-day in-hospital mortality (Fig. 3): when considered as a continuous variable we found an 8% relative risk increase per 50 mg/l increase in plasma CRP, range 3–13% (Hazard Ratio (HR) 1.08, 95%-CI 1.03, 1.13; p < 0.001, when considered as a dichotomized variable the observed relative risk increase was 28% in the group of CRP > median, range 7–53% (HR 1.28, 95%-CI 1.07, 1.53; p = 0.0065). Corresponding subgroup analyses within each SCAI stage are given in Appendix Table 3.
Association between plasma CRP concentrations, 30-day mortality and therapy response (MCS use) in patients with cardiogenic shock. CRP C-reactive protein, MCS use use of mechanical circulatory support device, HR hazard ratio per 50 mg/l CRP increase and CRP > vs. ≤ median, 95%-CI 95% confidence interval. Cox regression models adjusted for age, sex, acute myocardial infarction, lactate, pH, cardiopulmonary resuscitation (CPR), patient intubated due to index event and SCAI-stage
Systemic inflammation and therapy response
Patients with higher CRP concentrations had a lower 30-day mortality when treated with MCS [56.78% (95%-CI 47.28, 64.57%)] compared to those not treated with MCS [64.81% (95%-CI 59.11, 69.72%)]. Conversely, patients with lower CRP concentrations had a higher 30-day in-hospital mortality when treated with MCS [69.3% (95%-CI 61.05, 75.8%) vs. 55.13% (95%-CI 48.64, 60.8%)] (Fig. 2). For Kaplan–Meier curves see Appendix Fig. 5. In the adjusted analysis, elevated plasma CRP concentrations appeared to only be associated with a higher mortality risk in patients not treated with MCS compared to those treated with MCS, although the interaction was only statistically significant if plasma CRP concentration was considered as a dichotomized variable: relative risk increase of 50% with plasma CRP concentrations > median in patients without MCS use (HR 1.50, 95%-CI 1.21, 1.86; p < 0.001) vs. no significant risk increase with plasma CRP concentrations > median in patients with MCS use (HR 0.92, 95%-CI 0.67, 1.26; p = 0.59; p-interaction = 0.01; Fig. 3). Results looking at each device separately (VA-ECMO, Impella®, ECMELLA) replicated these findings and are given in Appendix Table 4.
Discussion
The key findings of our analysis aiming to investigate the role of systemic inflammation in patients with CS were, firstly, plasma CRP concentrations seem to be equally distributed in patients with higher vs. lower shock severity. Secondly, even after adjustment for variables representing patient, clinical and shock-specific characteristics, plasma CRP concentration was a strong predictor of 30-day in-hospital mortality risk. And thirdly, higher plasma CRP concentrations were only associated with higher mortality in patients not treated with MCS, but not in those treated with MCS, indicating that MCS use might mitigate the hazardous impact of systemic inflammation in CS.
CS is increasingly recognized as heterogenous in underlying disease mechanisms, severity and response to therapies [4, 14, 23, 24]. The pathophysiology of CS includes more than only the reduction of cardiac output but instead also involves factors such as microvascular disturbances and systemic inflammation, which in combination contribute to multi-organ failure [3, 15, 17]. While this systemic inflammation can be caused by bacterial infection, a sterile systemic inflammation, also known as systemic inflammatory response (SIRS), can especially be triggered by various other factors [15], most of which are common in CS, such as tissue hypoxia/damage [25, 26], myocardial pressure overload [27] and activation of the sympathetic system [28]. The involvement of systemic inflammation in CS is highlighted by biomarker studies reporting an increase in plasma concentrations of CRP and inflammatory cytokines such as IL-6 and TNFα in patients with CS [16, 29], being correlated with the severity of CS and linked to a poor prognosis [18, 19]. A recent study suggested SIRS was increasingly prevalent as the severity of CS increased and associated with higher in hospital mortality [13]; moreover, IL-6 was found to be an independent predictor of early mortality in patients with AMI-CS [18]. Also, CRP has been found to predict cardiovascular mortality in asymptomatic adults and patients with acute coronary syndrome (ACS) [30, 31].
Contrary to these findings and what one might expect in our study population plasma CRP concentrations were not correlated with shock severity based on the SCAI classification. One explanation of this discrepancy could be a more detailed adjustment within the scope of our analysis, especially as the statistical association dissolved with increasing adjustment variables. A further explanation could be, that these prior studies have focused primarily on patients with AMI-CS, whereas our study population included all etiology CS. Also, higher CRP concentrations showed a strong association with higher lactate concentrations which are directly linked to the extent of tissue hypoperfusion and therefore shock duration. We hypothesize, that a slow but progressive onset of CS leaves more time for the patients to develop higher CRP concentrations compared to patients with a highly acute and severe onset as commonly seen in AMI and CA cases. This also explains the higher rate of CPR, AMI and ventilation in the group with lower CRP levels. The association between CRP and lactate persisted despite adjustment including SCAI stage indicating systemic inflammation might be a separate and independent component affecting patients across all different SCAI stages.
Furthermore, this study identified a strong association between plasma CRP concentrations and 30-day in-hospital mortality in patients with CS. This effect was present even when accounting for the established predictors of mortality such as lactate [32] and CA [33] as well as severity of CS, using the SCAI shock classification [4]; and is consistent with above mentioned prior published literature. While the reduction of cardiac output undoubtedly represents the initial driving factor of CS, maladaptive compensatory mechanisms in dissociation from the initial cardiac damage, including a systemic inflammatory response, seem to be central in fueling the endless downward spiral leading to refractory CS. Therefore, these data further support the idea that the presence of systemic inflammation may be a clinically useful tool to identify higher risk patients and provide additional risk stratification within the SCAI staging criteria. One key advantage being, CRP is an easily accessible and widely used laboratory parameter in daily clinical practice that could serve as surrogate parameter for systemic inflammation.
In addition to prognostication, it is important to evaluate whether the benefit of select therapies differs based on the presence of systemic inflammation. In particular regarding the use of MCS in patients with systemic inflammation it has been questioned if restoring macro-circulatory hemodynamics is sufficient to halt the downward spiral of CS and is effective for raising blood pressure and allowing vasopressors to be weaned in a mixed cardiogenic-vasodilatory shock phenotype with inadequate peripheral vascular tone [23]; also complicated by the fact that MCS provide additional triggers for a dysregulated inflammatory response [34, 35]. Furthermore, adverse events associated with MCS use are frequent calling for careful patient selection [9, 36].
Data regarding CS management, especially MCS and systemic inflammatory response remain scarce. A previous study showed a reduction in IL-6 after MCS use and its association with survivorship [37], contrary to other findings reporting a reduction in IL-6 after MCS use regardless of the outcome [38]. In this study, we could show that elevated plasma CRP concentrations appeared to only be associated with higher mortality in patients not treated with MCS, but not in those treated with MCS. This may be affected by selection bias, as only patients with perceived benefit were chosen for MCS. Also, the argument could be made that the use of MCS reflects the severity of CS. In more severely ill patients not responding to treatment other predictors of mortality could be more important than CRP concentrations. However, the association remained after adjusting for severity of CS and other predictors of mortality. Also, the results of CRP measurements were not yet available when the decision for or against MCS was made.
Therefore, we hypothesize systemic inflammation may play a central role contributing to the downward spiral of CS. MCS use might mitigate this negative impact of systemic inflammation potentially by providing sufficient end-organ perfusion with reduction of metabolic derangements, inflammatory cytokines and oxidative stress which could alleviate further microcirculatory dysfunction caused by CS. Hence the argument could be made that aside from a causal and timely treatment of the underlying condition which is the utmost important element, a promptly use of MCS in patients with systemic inflammation is also important to contain the downward spiral of CS. The presence or absence of systemic inflammation, measured by CRP concentrations or other inflammatory markers, may one day play a role in therapeutic decision making regarding MCS in CS. In light of the recently published ECLS-shock trial [39] and associated metanalysis [40] by Thiele et al. it is important to recognize the calling for a careful review of the indication for VA-ECMO treatment as it demonstrated no benefit in 30-day mortality for AMI-CS patients treated vs. not treated with VA-ECMO. It would be interesting if our hypothesis could be validated in a substudy of the ECLS-shock trial looking at the mortality of the subpopulation with increased systemic inflammation.
Limitations
One major limitation of this study is its retrospective and monocentric study design which includes several factors: Firstly, the possibility of a selection bias as patients were identified via ICD-10 coding. This might have led to the inclusion of misdiagnosed patients who did not actually suffer from CS but other causes of shock. However, this is rather unlikely as a careful manual chart review by a physician was performed in all cases. Secondly, not all aspects of the SCAI classification were available (e.g., specific biomarkers or invasive hemodynamics) potentially leading to a misclassification regarding SCAI shock stage. Thirdly, the potential of missing data and unmeasured or unknown confounders preclude from establishing causal relationships.
In addition, we acknowledge systemic inflammation is the result of different inflammatory mechanisms and mediators and may not be represented holistically by the exclusive analysis of plasma CRP concentrations. Plasma CRP is a sensitive yet non-specific biomarker of systemic inflammation and induced under various conditions including infection, trauma and other inflammatory states such as autoimmune disease. This could lead to difficulties regarding a clear interpretation, especially in patients with additional infections where CRP concentrations may affect therapy and prognosis.
Our study merely describes associations and is not designed to investigate whether systemic inflammation plays a causal role in shock severity, 30-day mortality and therapy response. Larger multicentre prospective studies are necessary to confirm our findings and animal studies are necessary to establish causation between systemic inflammation and CS.
Conclusion
In patients with CS increased concentrations of plasma CRP are associated with a higher 30-day mortality regardless of shock severity; which is consistent with prior studies associating increased inflammatory markers with a poor prognosis. Furthermore, our results indicate that MCS use might mitigate this negative impact of systemic inflammation. Overall, plasma CRP is an easily accessible marker of systemic inflammation that could serve as independent predictor of outcomes in patients with CS. Also, CRP could potentially be considered for therapeutic decision making regarding the use of MCS in CS and calls for further research on this topic.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AMI:
-
Acute myocardial infarction
- CA:
-
Cardiac arrest
- CRP:
-
C-reactive protein
- CS:
-
Cardiogenic shock
- MCS:
-
Mechanical circulatory support
- PCI:
-
Percutaneous coronary intervention
- SCAI:
-
Society for Cardiovascular Angiography and Intervention
- SIRS:
-
Systemic inflammatory response
- VA-ECMO:
-
Veno-arterial extracorporeal membrane oxygenation therapy
References
Schrage B, Becher PM, Gossling A, Savarese G, Dabboura S, Yan I, Beer B, Soffker G, Seiffert M, Kluge S et al (2021) Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail 8:1295–1303. https://doi.org/10.1002/ehf2.13202
Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH et al (2014) Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 3:e000590. https://doi.org/10.1161/JAHA.113.000590
Krychtiuk KA, Vrints C, Wojta J, Huber K, Speidl WS (2022) Basic mechanisms in cardiogenic shock: part 1-definition and pathophysiology. Eur Heart J Acute Cardiovasc Care 11:356–365. https://doi.org/10.1093/ehjacc/zuac021
Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sorensen NA, Gossling A, Becher PM, Grahn H, Wagner T et al (2020) Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv 96:E213–E219. https://doi.org/10.1002/ccd.28707
Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA (2019) Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol 74:2117–2128. https://doi.org/10.1016/j.jacc.2019.07.077
Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S (2019) Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 40:2671–2683. https://doi.org/10.1093/eurheartj/ehz363
Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C et al (2017) PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 377:2419–2432. https://doi.org/10.1056/NEJMoa1710261
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G et al (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367:1287–1296. https://doi.org/10.1056/NEJMoa1208410
Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, CudemusDeseda G, Dabboura S, Eckner D et al (2020) Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation 142:2095–2106. https://doi.org/10.1161/CIRCULATIONAHA.120.048792
Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J et al (2017) Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 69:278–287. https://doi.org/10.1016/j.jacc.2016.10.022
Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M et al (2023) Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS Randomized Clinical Trial. Circulation 147:454–464. https://doi.org/10.1161/CIRCULATIONAHA.122.062949
Jentzer JC, Schrage B, Holmes DR, Dabboura S, Anavekar NS, Kirchhof P, Barsness GW, Blankenberg S, Bell MR, Westermann D (2021) Influence of age and shock severity on short-term survival in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care 10:604–612. https://doi.org/10.1093/ehjacc/zuaa035
Jentzer JC, Lawler PR, van Diepen S, Henry TD, Menon V, Baran DA, Dzavik V, Barsness GW, Holmes DR Jr, Kashani KB (2020) Systemic inflammatory response syndrome is associated with increased mortality across the spectrum of shock severity in cardiac intensive care patients. Circ Cardiovasc Qual Outcomes 13:e006956. https://doi.org/10.1161/CIRCOUTCOMES.120.006956
van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK et al (2017) Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 136:e232–e268. https://doi.org/10.1161/CIR.0000000000000525
Kohsaka S, Menon V, Lowe AM, Lange M, Dzavik V, Sleeper LA, Hochman JS, Investigators S (2005) Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med 165:1643–1650. https://doi.org/10.1001/archinte.165.14.1643
Cuinet J, Garbagnati A, Rusca M, Yerly P, Schneider AG, Kirsch M, Liaudet L (2020) Cardiogenic shock elicits acute inflammation, delayed eosinophilia, and depletion of immune cells in most severe cases. Sci Rep 10:7639. https://doi.org/10.1038/s41598-020-64702-0
Johansson PI, Stensballe J, Ostrowski SR (2017) Shock induced endotheliopathy (SHINE) in acute critical illness—a unifying pathophysiologic mechanism. Crit Care 21:25. https://doi.org/10.1186/s13054-017-1605-5
Geppert A, Dorninger A, Delle-Karth G, Zorn G, Heinz G, Huber K (2006) Plasma concentrations of interleukin-6, organ failure, vasopressor support, and successful coronary revascularization in predicting 30-day mortality of patients with cardiogenic shock complicating acute myocardial infarction. Crit Care Med 34:2035–2042. https://doi.org/10.1097/01.CCM.0000228919.33620.D9
Kataja A, Tarvasmaki T, Lassus J, Sionis A, Mebazaa A, Pulkki K, Banaszewski M, Carubelli V, Hongisto M, Jankowska E et al (2021) Kinetics of procalcitonin, C-reactive protein and interleukin-6 in cardiogenic shock—insights from the CardShock study. Int J Cardiol 322:191–196. https://doi.org/10.1016/j.ijcard.2020.08.069
Brechot N, Hajage D, Kimmoun A, Demiselle J, Agerstrand C, Montero S, Schmidt M, Luyt CE, Lebreton G, Hekimian G et al (2020) Venoarterial extracorporeal membrane oxygenation to rescue sepsis-induced cardiogenic shock: a retrospective, multicentre, international cohort study. Lancet 396:545–552. https://doi.org/10.1016/S0140-6736(20)30733-9
Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP et al (2019) SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 94:29–37. https://doi.org/10.1002/ccd.28329
Schrage B, Sundermeyer J, Beer BN, Bertoldi L, Bernhardt A, Blankenberg S, Dauw J, Dindane Z, Eckner D, Eitel I et al (2023) Use of mechanical circulatory support in patients with non-ischaemic cardiogenic shock. Eur J Heart Fail. https://doi.org/10.1002/ejhf.2796
Lawler PR, Mehra MR (2018) Advancing from a “hemodynamic model” to a “mechanistic disease-modifying model” of cardiogenic shock. J Heart Lung Transplant 37:1285–1288. https://doi.org/10.1016/j.healun.2018.07.009
Schrage B, Weimann J, Dabboura S, Yan I, Hilal R, Becher PM, Seiffert M, Bernhardt AM, Kluge S, Reichenspurner H et al (2020) Patient characteristics, treatment and outcome in non-ischemic vs. ischemic cardiogenic shock. J Clin Med. https://doi.org/10.3390/jcm9040931
Timmermans K, Kox M, Scheffer GJ, Pickkers P (2016) Danger in the intensive care unit: damps in critically ill patients. Shock 45:108–116. https://doi.org/10.1097/SHK.0000000000000506
Selejan S, Poss J, Walter F, Hohl M, Kaiser R, Kazakov A, Bohm M, Link A (2012) Ischaemia-induced up-regulation of Toll-like receptor 2 in circulating monocytes in cardiogenic shock. Eur Heart J 33:1085–1094. https://doi.org/10.1093/eurheartj/ehr377
Chen WY, Hong J, Gannon J, Kakkar R, Lee RT (2015) Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc Natl Acad Sci USA 112:7249–7254. https://doi.org/10.1073/pnas.1424236112
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES (2000) The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52:595–638
Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T (2021) Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol 12:746494. https://doi.org/10.3389/fphys.2021.746494
Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Braunwald E, Gibson CM (2002) Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy- Thrombolysis in Myocardial Infarction 18 trial)substudy. J Am Coll Cardiol 40:1761–1768. https://doi.org/10.1016/s0735-1097(02)02484-1
Sheikh AS, Yahya S, Sheikh NS, Sheikh AA (2012) C-reactive protein as a predictor of adverse outcome in patients with acute coronary syndrome. Heart Views 13:7–12. https://doi.org/10.4103/1995-705X.96660
Jentzer JC, Schrage B, Patel PC, Kashani KB, Barsness GW, Holmes DR Jr, Blankenberg S, Kirchhof P, Westermann D (2022) Association between the acidemia, lactic acidosis, and shock severity with outcomes in patients with cardiogenic shock. J Am Heart Assoc 11:e024932. https://doi.org/10.1161/JAHA.121.024932
Jentzer JC, Henry TD, Barsness GW, Menon V, Baran DA, Van Diepen S (2020) Influence of cardiac arrest and SCAI shock stage on cardiac intensive care unit mortality. Catheter Cardiovasc Interv 96:1350–1359. https://doi.org/10.1002/ccd.28854
Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF (2016) The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 20:387. https://doi.org/10.1186/s13054-016-1570-4
Frerou A, Lesouhaitier M, Gregoire M, Uhel F, Gacouin A, Reizine F, Moreau C, Loirat A, Maamar A, Nesseler N et al (2021) Venoarterial extracorporeal membrane oxygenation induces early immune alterations. Crit Care 25:9. https://doi.org/10.1186/s13054-020-03444-x
Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Mullerleile K, Colombo A et al (2017) Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 19:404–412. https://doi.org/10.1002/ejhf.668
Diakos NA, Thayer K, Swain L, Goud M, Jain P, Kapur NK (2021) Systemic inflammatory burden correlates with severity and predicts outcomes in patients with cardiogenic shock supported by a percutaneous mechanical assist device. J Cardiovasc Transl Res 14:476–483. https://doi.org/10.1007/s12265-020-10078-5
Andrie RP, Becher UM, Frommold R, Tiyerili V, Schrickel JW, Nickenig G, Schwab JO (2012) Interleukin-6 is the strongest predictor of 30-day mortality in patients with cardiogenic shock due to myocardial infarction. Crit Care 16:R152. https://doi.org/10.1186/cc11467
Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, Lehmann R, Eitel I, Graf T, Seidler T et al (2023) Extracorporeal life support in infarct-related cardiogenic shock. N Engl J Med. https://doi.org/10.1056/NEJMoa2307227
Zeymer U, Freund A, Hochadel M, Ostadal P, Belohlavek J, Rokyta R, Massberg S, Brunner S, Lusebrink E, Flather M et al (2023) Venoarterial extracorporeal membrane oxygenation in patients with infarct-related cardiogenic shock: an individual patient data meta-analysis of randomised trials. Lancet. https://doi.org/10.1016/S0140-6736(23)01607-0
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BS received speaker fees from Abiomed, AstraZeneca and Abbott; and research support from Abiomed, the German Research Foundation and the Else Kröner-Fresenius-Stiftung. PK was partially supported by European Union AFFECT-AF (grant agreement 847770), and MAESTRIA (grant agreement 965286), British Heart Foundation (PG/17/30/32961; PG/20/22/35093; AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), Deutsche Forschungsgemeinschaft (Ki 509167694), and Leducq Foundation. PK receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last three years. PK is listed as inventor on two issued patents held by University of Hamburg (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). FJB reports grants from Daiichi Sankyo, Novartis, Pfizer, and Sanofi, non-financial support from Abbott, ASAHI INTECC, and Inari Medical, personal fees from Amgen and Novartis outside of the submitted work. The other authors have no conflicts of interest to declare.
Appendices
Appendices
See Tables 2, 3, 4 and Figs. 4 and 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dettling, A., Weimann, J., Sundermeyer, J. et al. Association of systemic inflammation with shock severity, 30-day mortality, and therapy response in patients with cardiogenic shock. Clin Res Cardiol 113, 324–335 (2024). https://doi.org/10.1007/s00392-023-02336-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02336-8