Abstract
Since the first description of apical hypertrophic cardiomyopathy (ApHCM) in 1976, contrasting information from all over the world has emerged regarding the natural history of the disease. However, the recommended guidelines on hypertrophic cardiomyopathy (HCM) pay a cursory reference to ApHCM, without ApHCM-specific recommendations to guide the diagnosis and management. In addition, cardiologists may not be aware of certain aspects that are specific to this disease subtype, and a robust understanding of specific disease features can facilitate recognition and timely diagnosis. Therefore, the review covers the incidence, pathogenesis, and characteristics of ApHCM and imaging methods. Echocardiography and cardiovascular magnetic resonance imaging (CMR) are the most commonly used imaging methods. Moreover, this review presents the management strategies of this heterogeneous clinical entity. In this review, we introduce a novel transapical beating-heart septal myectomy procedure for ApHCM patients with a promising short-time result.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is mostly an autosomal dominant disease characterized predominantly by the detection of left ventricular (LV) hypertrophy in the absence of another cardiac, systemic, or metabolic disease. According to the segments of LV hypertrophy, HCM can be classified into basal (also called classic HCM), midventricular, and apical (Fig. 1). Apical hypertrophic cardiomyopathy (ApHCM) is characteristic of hypertrophy predominantly involving the LV apex with giant negative T waves in the electrocardiogram and a “spade-like” configuration of the LV cavity on the LV ventriculogram. However, the current guidelines [1, 2] on HCM pay a cursory reference to ApHCM, without ApHCM-specific recommendations to guide the diagnosis and management. Thus, this review summarizes the epidemiology, pathophysiology, and clinical characteristics of ApHCM, while also highlighting knowledge of diagnostic techniques and management strategies. In this review, we introduce a novel transapical beating-heart septal myectomy procedure for ApHCM patients with a promising short-time result.
Epidemiology
Hypertrophic cardiomyopathy is estimated to affect 1 out of 500 people. The ApHCM has a different prevalence in cohorts of patients with HCM, which is relatively higher in Asian ethnicity (Table 1). It is considered less common (8%) in Europe and North America with a majority (84%) of White race [3]. However, ApHCM may occur more frequently in Asian race, in whom it is seen in up to 40% of HCM patients (21% in China [4], 30% in Japan [5], and 38% in Korea [6]) of patients with HCM.
ApHCM is worldwide in distribution and affects males more frequently than females, with male-to-female ratios typically 1.6 to 2.8:1 [7]. Most commonly diagnosed in midlife [3], the early- and late-onset expressions are also known to occur.
Genetics
HCM is a genetic disorder of the myocardium, inherited in an autosomal dominant pattern with variable expressivity and age-related penetrance. So far, over 1500 mutations in 15 or more genes encoding the sarcomeric proteins and related myofilament elements have been reported in approximately half of the patients [8,9,10]. Mutations in the beta-myosin heavy chain (MYH7) and myosin binding protein C (MYBPC3) are most common, accounting for up to 50% of familial HCM patients [8, 11, 12]. About 5% of patients have at least two mutations and about 30% have genetically unexplained diseases.
Similar to classic HCM, although ApHCM involves genetic mutations (autosomal dominant inheritance pattern), implicating a role for genetics in the development of this morphological pattern of hypertrophy [13], this has been poorly explained. Genetic analyses based on studies of the small-sized population with ApHCM identified a predilection for ACTC1 (cardiac a-actin) mutations, indicating direct causality of ApHCM with cardiac actin mutations like Glu101Lys and association of ApHCM with ACTC E101K mutation [14, 15]. However, in the largest cohort of patients with genetic testing for HCM to date, the apical form was most commonly associated with mutations in MYBPC3 (6; 33%) and MYH7 (6; 33%), similar to the general HCM cohort [16]. In this study, the yield of genetic testing in ApHCM is relatively low (only 25% positivity).
Classification
Morphologically ApHCM can be subclassified into three subtypes [17]: pure, mixed, and relative ApHCM (Supplementary Fig. 1). Pure AHCM presented with hypertrophy that was confined to the apex. Mixed ApHCM displayed both apical and septal hypertrophy but with the thickest apex. Finally, relative ApHCM is believed to be an early ApHCM phenotype. Individuals with relative ApHCM do not meet conventional diagnostic criteria for ApHCM but have similar imaging findings with the pure type. Relative ApHCM can be diagnosed when electrocardiography shows characteristic precordial T-wave inversion and cardiac imaging techniques show apical wall thickness exceeding basal wall thickness, although failing to reach the cutoff of wall thickness ≥ 15 mm [18].
Relative ApHCM was originally considered entirely benign [17], but Flett et. al [18] suggested associated pathology with left atrial dilatation, apical obliteration, apical aneurysm, and myocardial scar (Fig. 2). In another study, given the absence of other causes of this abnormality, relative apical hypertrophy appeared to be the only explanation for giant T-wave (≥ 1 mV) inversion [19]. Thus, relative ApHCM may solely represent the early course of the disease that would with time progress to overt ApHCM and meet the conventional criteria.
ECG and CMR in relative ApHCM. ECG demonstrates giant negative T-wave inversion in precordial leads and voltage criteria for LV hypertrophy (A). CMR demonstrates a thickened LV apex (13.5 mm) with relative but not absolute apical hypertrophy in diastole (B), apical cavity obliteration in systole (C), a small LV apical aneurysm (D), and the presence of LGE in the apex (E). ApHCM apical hypertrophic cardiomyopathy, CMR cardiac magnetic resonance imaging, ECG electrocardiogram, LGE late gadolinium enhancement, LV left ventricular
Pathophysiology
Cavity obliteration
Apical cavity obliteration occurs usually in patients with ApHCM. The degree of apical cavity obliteration is measured by the ratio of obliteration to cavity, which is defined as the end-systolic length of apical obliteration to the end-systolic height of the LV cavity [20]. Apical hypertrophy is an ongoing process and causes cardiac function to deteriorate later on. Thus, the effect of apical obliteration can strengthen gradually as the LV cavity size decreases, which is proved by the ever-increasing adverse cardiovascular events identified during the follow-up [20]. In addition, severe apical cavity obliteration has been associated with myocardial ischemia at the obliterated area [21].
Hypertrophy involving the mid-ventricular segments can typically result in mid-ventricular obstruction (MVO) with cavity obliteration (MVOCO) [22], which is a complication of mixed rather than pure ApHCM (Supplementary Fig. 2). Indeed, MVO is related to the presence of apical aneurysms [23], the reduced survival and increased risk of ventricular arrhythmias [24]. In severe cases, MVOCO lasts into diastole and is associated with a paradoxical diastolic jet flow (PJF), which indicates an increased risk of thromboembolism, arrhythmias, and a worse prognosis [21].
Diastolic dysfunction
LV wall thickening, especially of the apex, results in a decrease in the diastolic volume of the LV, consequently leading to the reduction of end-diastolic volume and cardiac output. Myocardial hypertrophy results in myocardial ischemia along with the formation of interstitial fibrosis, both responsible for increased chamber stiffness. Therefore, altered ventricular load with high LV filling pressures, nonuniformity in myocardial contraction and relaxation are common in patients with ApHCM, each of which could contribute to advanced diastolic dysfunction [6, 25]. ApHCM patients are reported to have diastolic dysfunction as evidenced by a dilated left atrium (LA) and longer isovolumic relaxation time, suggesting that local apical hypertrophy could affect global diastolic function [6, 26].
Diastolic dysfunction is the core of several clinical manifestations of ApHCM, including dyspnea, exercise intolerance, and pulmonary edema. With impairment in ventricular myocardial relaxation, greater dependency on the atrial systole for ventricular filling may occur, leading to dilatation of the LA, which increases the risk of atrial fibrillation (AF) episodes or other arrhythmias. Therefore, the LA enlargement could reflect the severity of diastolic dysfunction and predict poor prognosis in ApHCM.
Apical aneurysms
The apical aneurysm is defined as a discrete, thin-walled, and dyskinetic or akinetic segment of the most distal portion of the LV [27]. The incidence of the apical aneurysm has been reported to account for approximately 2–5% of HCM [28,29,30], although it is a more common (20–30%) finding in the ApHCM subgroup [18, 25, 30]. A cue to their presence is the persistence of apical blood pooling distal to the point of apical systolic cavity obliteration. The aneurysm size is defined as the maximum transverse dimension measured by CMR or echocardiography in the four-chamber view at the end-systole and is further characterized as small (< 2 cm), medium (2–4 cm), and large (> 40 mm) [31].
There are only a few reports focusing on the dynamic process of apical aneurysm formation. In a recent study of 72 patients with ApHCM, the course of dynamic change of apical aneurysm formation was recorded by using at least two CMR examinations, indicating that the formation of apical aneurysm may follow four stages [32]. (1) It starts with apical systolic cavity obliteration or an apical slit due to the increased thickness of the apical wall. (2) Then the apical slit broadens in systole due to the combination of proximal obstruction of the hypertrophied segments and reduced contractile movement of the distal hypertrophy. (3) The apical slit subsequently develops into an apical outpouching. (4) Finally, the apical aneurysm forms. Yang et al. [32] suggested that the interval between the two stages was variable, but the mean interval from ApHCM to the development of apical aneurysm would take over 10 years, which was consistent with the findings of previous reports [33]. However, further studies are needed to identify a more detailed process of apical aneurysm formation.
The clinical course of ApHCM patients with apical aneurysms is variable but overall proved to be unfavorable [27]. Previous studies have shown that apical aneurysm is associated with a higher risk of adverse cardiovascular events, including cardiovascular death, ventricular tachycardia, and AF [28, 31]. Moreover, the apical aneurysm carries the risk of apical thrombosis and thromboembolic stroke [34]. Rowin et al. [28] reported that the appropriate ICD therapy rate for primary prevention was 4.0%/year in patients with apical aneurysm, about fivefold greater than the sudden cardiac death (SCD) event rate in those without apical aneurysm.
Clinical characteristics
Clinical presentation
The patients with ApHCM do not have any pathognomonic clinical symptoms and their main complaints are unspecific, leading to late diagnosis [35]. The most common presenting symptoms are chest pain, palpitations, dyspnea, as well as syncope [4]. In a series of 208 consecutive patients with AHCM, chest discomfort (defined as chest tightness or chest pain) was reported by 91.8% of patients, palpitations by 30.8%, dyspnea on exertion by 10.6%, presyncope/syncope by 7.2% [36]. Yin and colleagues [4] reported that 42.1% of ApHCM patients had a history of hypertension, 22.2% with heart failure, 13.5% with diabetes, and 7.1% with ischemic heart disease. ApHCM also occasionally manifests as morbid events such as atrial fibrillation, ventricular fibrillation, and sudden cardiac death [7, 37].
Prognosis
The ApHCM was originally thought to have a favorable long-term prognosis. With a mean follow-up of 13.6 ± 8.3 years from presentation, the North American cohort of 105 mildly symptomatic patients with ApHCM showed low mortality of 10.5% (11/105) and annual mortality of 0.6% [38]. In another study from China, 3 (1.6%) of 208 patients with ApHCM died during a mean follow-up of 8.0 ± 3.5 years, and the annual mortality was 0.3% [5]. However, Klarich et al. [7] observed a cohort of 193 patients for more than 20 years and found a worse 20-year survival in patients with ApHCM versus expected for the normal population (47% versus 60%). Among a referral-based cohort of 126 patients with ApHCM [4], 34 (27%) experienced adverse events during a mean follow-up of 8.0 ± 3.0 years, including (1) cardiac death events (4.8%); (2) myocardial infarction unrelated to coronary artery disease (2.4%); (3) progressive heart failure with an increase of at least one NYHA functional class (13.5%); (4) appropriate implantable cardioverter-defibrillator (ICD) interventions for ventricular tachycardia or ventricular fibrillation (5.6%); (5) new-onset AF (6.3%); (6) and thromboembolic stroke (4.0%).
In an observational study of 208 Chinese patients, only age > 60 years, left atrial diameter > 36 mm, and NYHA class III were independently associated with increased risk of death [36]. A coexisting CAD was identified as a strong risk factor for survival in patients with ApHCM [39]. In addition, the distribution of hypertrophy was reported to influence survival; the mixed form and more severe hypertrophy were associated with a worse prognosis [5]. Further, patients with complete end-systolic cavity obliteration and apical aneurysm have been found to have higher cardiovascular morbidity [20, 27].
Diagnosis
Diagnostic criteria
ApHCM is described as an electrocardiographic pattern of giant negative T-waves (≥ 1 mV) together with a “spade-like configuration” of the LV cavity on the left ventriculography. [40] With advances in imaging techniques, the current definition mainly relies on demonstrating LV hypertrophy predominating in the LV apex, with the apical wall thickness ≥ 15 mm and a ratio of maximal apical to posterior wall thickness ≥ 1.5, based on echocardiography or CMR [38]. However, recent studies used the diagnostic criteria as inappropriate apical hypertrophy (loss of apical tapering and apical thickness exceeding basal thickness) but < 15 mm, and other characteristics (apical cavity obliteration, or apical aneurysm) [30], highlighting a novel trend of using a lower diagnostic cutoff.
Electrocardiography
The trademark electrocardiographic finding for ApHCM is LV hypertrophy with giant T waves, which are defined as T-waves ≥ 1 mV in any electrocardiography lead. These giant T waves are dynamic and will change over time, or even disappear, as a part of the natural history of ApHCM [41]. The depth of T waves varies among patients, in a study of 208 ApHCM patients, Yan et al. [38] reported that 60 (28%) patients had giant T-waves, but only 11% of ApHCM patients had giant T waves in a series from Mayo Clinic [7]. Although giant T-waves in the setting of LV hypertrophy are considered pathognomonic for ApHCM, it is unlikely to rule out other causes of ST-T wave abnormalities, such as other types of HCM, coronary heart disease, medication effect (e.g., digoxin), and neurological diseases (e.g., subarachnoid hemorrhage). Considering the existence of numerous potential differential diagnoses, it is necessary to conduct a comprehensive evaluation through cardiac imaging to confirm the diagnosis of ApHCM.
Another suggested examination is ambulatory 24- or 48-h electrocardiography, which is crucial to detect non-sustained ventricular tachycardia (NSVT), AF, and ventricular fibrillation. Eriksson et al. [38] showed that Holter monitor recordings revealed NVST in 20 patients (23%) with ApHCM, which was found to be correlated with the presence of fibrosis. Several studies have reported that the prevalence of AF was 11–17% [4, 5]. LA enlargement secondary to LV diastolic dysfunction in patients with ApHCM can predict subsequent AF, which is prognostically adverse [42].
Echocardiography
In contemporary clinical practice, transthoracic echocardiography (TTE) is the first-line imaging modality for the evaluation of ApHCM because of its noninvasiveness, wide availability, and low cost [43, 44]. A comprehensive echocardiographic examination should involve the assessment of (1) the distribution and magnitude of LV hypertrophy; (2) the presence of cavity obliteration and apical aneurysm; (3) obstruction at any level in the LV; (4) systolic and diastolic function; (5) and mitral apparatus and papillary muscle abnormalities [43,44,45]. ApHCM is diagnosed as having asymmetrical LV hypertrophy confined predominantly to the LV apex, an apical wall thickness ≥ 15 mm, and a ratio of apical to posterior wall thickness ≥ 1.5 [46, 47]. However, as the apical cap is the thinnest part of the LV, the cut-off value (15 mm) may be less in the presence of the accompanying findings [17, 48]. A lower threshold can be used to diagnose ApHCM when the clinical presentation and other imaging characteristics lend themselves to the diagnosis of ApHCM [30]. The apical wall thickness should be carefully assessed as foreshortening on segment measurements can lead to an overestimation of apical thickness by up to almost 30% [49].
Local dysfunction involves the apical segments, manifested as hypokinetic, akinetic, or even dyskinetic, especially in the presence of an apical aneurysm [34]. LV twist is significantly decreased due to a reduction in apical rotation, leading to the loss of early diastolic suction and eventually diastolic dysfunction in ApHCM [50]. In ApHCM patients with MVOCO, diastolic intraventricular gradient often exists, manifesting as a directed flow of apex to base during early diastole—the PJF phenomenon, [23] which is associated with a high risk of systemic embolism, perfusion abnormalities, and ventricular arrhythmias.
Because of frequent difficulties in visualizing the apical endocardium, 2D echocardiography may result in misdiagnosis, as it was reported that echocardiography failed to diagnose ApHCM in 31.7% of patients [36]. Therefore, there is a need for an alternative imaging technique, and contrast echocardiography has been recommended as an alternative when 2D images are suboptimal [51]. The administration of echocardiographic contrast allows the excellent visualization of LV morphology and the presence of an apical aneurysm.
Cardiac magnetic resonance imaging
Another useful examination is CMR, which provides high-resolution images and may detect early ApHCM phenotypes better than echocardiography [17]. The advantage of CMR is the complete, unrestricted coverage of LV morphology [52], so it can accurately determine the site and extent of hypertrophy and the presence of apical aneurysm [44]. Currently, the use of CMR is standard practice for a comprehensive evaluation in patients with HCM, especially when ApHCM is suspected [22].
By utilizing the inherent magnetic properties of different tissues and the distribution of gadolinium-based contrast agents, CMR can accurately quantify and visualize the patterns of dense focal extracellular matrix deposition as seen in replacement fibrosis by late gadolinium enhancement (LGE). In several studies of ApHCM patients imaged with CMR, LGE is reported in up to 65–80% of cases [4, 31]. It is reported that LGE is strongly associated with clinical severity in terms of severe symptoms, disease progression, and poor prognosis [4, 53]. In addition, a recent study demonstrated that ApHCM patients with extensive LGE (≥ 15% of LV mass) tended to suffer adverse events compared with those with LGE < 15% [31].
CMR is particularly effective for delineating the presence of apical aneurysm, which is a prognostic indicator for ApHCM [54]. In a recent study, echocardiography (without contrast use) can only correctly identify 29% (9/31) of apical aneurysms in ApHCM patients, and most misdiagnosed cases were small aneurysms [31]. Apical aneurysms may present as dyskinesis or akinesis usually with transmural scarring on LGE, and are associated with adverse outcomes [55]. In addition, apical thrombi can be revealed with CMR, which may be overlooked with non-contrast echocardiography [28].
Cardiac computed tomography and angiography
Although not used commonly, cardiac computed tomography (CT) may be considered for diagnosis when echocardiography is technically limited and CMR imaging is contraindicated or unavailable. Cardiac CT provides excellent spatial resolution allowing for clear definition of LV structure (including hypertrophy pattern, wall thickness, detection of subaortic membrane, and intracardiac thrombus) and function [54, 56]. Moreover, in ApHCM patients with symptoms or evidence of myocardial ischemia, coronary CT is recommended and can detect the stenosis of coronary arteries with high sensitivity and specificity [57, 58]. Coronary CT is recommended for patients with a low to intermediate risk of CAD, which may contribute to early diagnosis of patients presenting chest pain [59].
Coronary angiography is usually performed in patients who are scheduled for apical myectomy and have risk factors for coronary atherosclerosis. With the existence of extensive CAD, the surgical strategy would alter to apical myectomy combined with coronary artery bypass grafting.
Management strategies
In the absence of large randomized trials, therapy in patients with ApHCM is largely based on the management of classic HCM to improve functional capacity, reduce symptoms, and prevent disease progression. However, due to the differences in pathophysiology and clinical characteristics between classic HCM and ApHCM, the management of ApHCM may differ to some extent from the classic HCM (Table 2).
Medical therapy
In ApHCM, beta-blockers are usually the initial drugs of choice and are often employed to reduce the prevalence of non-sustained ventricular arrhythmias. Their use is associated with (1) decreased heart rate response to exercise; (2) improved diastolic function; (3) relief of chest discomfort and dyspnea by reducing myocardial oxygen demand; and (4) decreased midventricular gradients with the existence of MVO. Despite these advantages, whether treatment with beta-blockers ultimately impacts outcomes in ApHCM patients remains undefined and needs to be verified in future studies. In general, non-vasodilatory beta-blockers are preferred to avoid exacerbating gradients in ApHCM patients with MVO [60].
The beneficial effects of verapamil and diltiazem are mediated by their negative inotropic and chronotropic mechanism, resulting in reduced chest pain, prolonged LV filling time in diastole, and improved myocardial perfusion [61]. Isolated refractory chest pain may be difficult to control if high doses of β-blockers or non-dihydropyridine calcium blockers are not aggressively used. The medication dosage should be titrated to effectiveness with monitoring for atrioventricular conduction block or bradycardia, especially in the combined usage of β-blockers and calcium channel blockers.
Low-dose loop or thiazide diuretics can be used to improve dyspnea and reduce volume overload in ApHCM patients. To prevent hypovolemia and hypotension, it is necessary to cautiously use any of these diuretics, usually by intermittent dosing as needed or chronic low-dose treatment.
Several studies aimed at the anti-fibrotic and antihypertrophic approaches targeting the renin–angiotensin–aldosterone system (RAAS), provide an additional treatment option for ApHCM. The benefit of RAAS inhibition on symptoms and mortality is assumed, and patients with heart failure and reduced LVEF are recommended to be treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, in line with the 2021 ESC Guidelines for the management of chronic heart failure [62]. A small randomized, double-blind, placebo-controlled trial in twenty patients with non-obstructive HCM showed that losartan slowed the progression of myocardial hypertrophy and fibrosis in patients with non-obstructive HCM [63]. However, in a larger randomized study of 133 patients with obstructive or non-obstructive HCM, treatment with losartan had no effect on cardiac function or exercise capacity compared with placebo [64], and challenged the generally held view that angiotensin II receptor blockers reduced cardiac hypertrophy [65].
Mavacamten is a selective allosteric inhibitor of cardiac myosin ATPase specifically developed to target the underlying pathophysiology of HCM by reducing actin–myosin cross-bridge formation [66, 67], thereby reducing contractility and improving myocardial energetics [68]. Although mavacamten in patients with symptomatic non-obstructive HCM was associated with a significant dose-dependent reduction in N-terminal pro-B-type natriuretic peptide (NT-proBNP), there was no significant impact of mavacamten on symptoms or functional capacity [69]. In a more recent randomized phase 3 trial, mavacamten markedly improved exercise capacity, NYHA functional class, and health status in patients with obstructive HCM [70]. Therefore, this agent might be only beneficial for ApHCM patients with MVO, but further studies are needed to confirm the benefit.
Implantable cardiac defibrillator
There are currently no randomized studies to guide the insertion of an implantable cardiac defibrillator (ICD) specifically for ApHCM. Over the past decades, a large number of studies have focused on identification of the clinical risk markers. Maron et al. [71] have recently evolved the American Heart Association guidance for ICDs by proposing new criteria for HCM patients, which includes new high-risk markers such as systolic dysfunction (EF < 50%), extensive LGE on CMR occupying ≥ 15% of LV mass by quantification or visual estimation, and the LV apical aneurysm of any size [71]. This risk stratification seems more sensitive than the ESC guidance [2] in predicting patients with ApHCM under the risk of SCD and indicates significant progress in understanding more personalized risk factors.
The current SCD risk markers, obtained from personal and family history, and noninvasive examinations (including echocardiography, ambulatory electrocardiogram, and CMR), can be used to estimate the risk level of ApHCM patients and to determine those most likely to benefit from primary prophylaxis of ICD therapy. It is reasonable to implant an ICD for ApHCM patients with ≥ 1 major SCD risk factor [1], including (1) family history of HCM-related SCD; (2) massive LV hypertrophy; (3) unexplained syncope; (4) NSVT on the ambulatory monitor; (5) LV systolic dysfunction (EF < 50%); (6) Extensive LGE on CMR imaging; (7) LV apical aneurysm (Table 3). Although the potential risk factors (MVOCO, PJF, AF, etc.) are not currently supported by a large body of evidence linking the risk factor to sudden death, these serve in the risk arbitration of SCD in ApHCM.
Alcohol septal ablation and surgery
Unlike classic HCM, the absence of LV outflow tract obstruction can render alcohol septal ablation (ASA) in ApHCM unwarranted. However, some case studies have highlighted its potential role in symptomatic patients with MVO, as it may improve symptoms and reduce intraventricular gradients [72]. Therefore, in ApHCM patients with MVO and at high risk for surgery, ASA is considered in the hands of experienced surgeons. In addition, it was reported that ASA eliminated VT in patients with ApHCM after catheter ablation failed to abolish VTs [73]. As ASA may produce substantial infarction and harm ventricular function, it should be limited to highly selected patients.
According to the 2020 AHA/ACC guideline, apical myectomy can be performed by experienced surgeons at comprehensive centers in highly selected ApHCM patients with severe symptoms and lifestyle limitations (diastolic heart failure, NYHA class 3–4 dyspnea) despite maximal medical therapy, and with preserved EF and small LV cavity [1]. Transapical myectomy has been reported to improve functional status and enlarge the LV cavity as well as stroke volume [74]. In brief, the operation is performed through a median sternotomy with cardiopulmonary bypass. The apical incision is made lateral to and far enough from the left anterior descending coronary artery so that closure will not compromise the vessel. After identification of the papillary muscles, initial myectomy is performed along the ventricular septum and additional resection along the free wall can be performed to enlarge the LV cavity. In addition, the prominent papillary muscles can be shaved to further increase LV volume. If an apical aneurysm is present, it will be repaired. The apical incision and its closure produce an area of akinesis, but this is tiny and does not result in significant systolic dysfunction [75]. Concomitant transaortic myectomy is performed in those with symptomatic MVO, as it may reduce intraventricular gradients and improve heart failure symptoms [76].
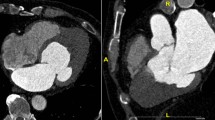
In a recent study from the Mayo Clinic, 113 symptomatic patients with ApHCM and advanced heart failure underwent transapical myectomy [77], 76% (32/41) of patients reported improvement in general health during a median follow-up of 5.2 (3.2–10.1) years. The survival of patients receiving apical myectomy appears better than those with HCM listed for cardiac transplantation, with the overall estimated 1-, 5-, and 10-year survivals of 96, 87, and 74%, respectively [77]. In addition, a novel transapical beating-heart septal myectomy (TA-BSM) procedure has been performed in obstructive HCM patients through the left intercostal incision with no need for cardiopulmonary bypass [78]. In a recent clinical trial, 46 (97.9%) of 47 patients with obstructive HCM showed improved functional status and significant relief of obstruction at 3 months follow-up [78]. Therefore, patients with ApHCM may also benefit from the TA-BSM procedure. The surgical results of ApHCM patients were satisfactory with significantly enlarged LV cavity (Fig. 3) and increased stroke volume. These preliminary findings, however, remain to be confirmed over a longer follow-up period in a larger patient population.
Transthoracic echocardiography (A, four-chamber; B, two-chamber) and cardiac magnetic resonance (C, four-chamber; D, two-chamber) in a patient with ApHCM at baseline. Note the increased thickness of the apex and small end-diastolic volume of the LV. Transthoracic echocardiography (E, four-chamber; F, two-chamber) and cardiac magnetic resonance (G, four-chamber; H, two-chamber) in the same patient one year after TA-BSM. Note the significantly reduced thickness of the apex and the increased end-diastolic volume of the LV. ApHCM apical hypertrophic cardiomyopathy, LV left ventricle, TA-BSM transapical beating-heart septal myectomy
Conclusions
ApHCM is a complex subset of HCM that is highly heterogeneous in its clinical and pathological profile. ApHCM presents special characteristics in epidemiology, pathophysiology, diagnosis, and therapy compared with more commonly detected and better understood classic HCM. Although ApHCM is relatively rare, patients with non-specific symptoms, such as chest pain, dyspnea on exertion, or syncope, should be carefully examined for the presence of ApHCM. Contemporary echocardiography provides certainty to the morphology of ApHCM that characterizes the disease phenotype, including MVOCO, diastolic dysfunction, and apical aneurysm. In addition, CMR can accurately detect apical aneurysms and identify regions of LGE. Similar to classic HCM, patients with ApHCM should undergo SCD risk assessment to determine whether to implant ICD. Transapical myectomy can be performed in a selected group of patients with good long-term results. In addition, the TA-BSM procedure is a novel therapy for ApHCM patients with minimal trauma and favorable early outcomes. These preliminary findings, however, remain to be confirmed over a longer follow-up period in a larger patient population.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P et al (2020) 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol 76(25):e159–e240
Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F et al (2014) 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35(39):2733–2779
Neubauer S, Kolm P, Ho CY, Kwong RY, Desai MY, Dolman SF et al (2019) Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM Registry. J Am Coll Cardiol 74(19):2333–2345
Yin Y, Hu W, Zhang L, Wu D, Yang C, Ye X (2022) Clinical, echocardiographic and cardiac MRI predictors of outcomes in patients with apical hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 38(3):643–651
Kubo T, Kitaoka H, Okawa M, Hirota T, Hoshikawa E, Hayato K et al (2009) Clinical profiles of hypertrophic cardiomyopathy with apical phenotype–comparison of pure-apical form and distal-dominant form. Circ J 73(12):2330–2336
Moon J, Shim CY, Ha JW, Cho IJ, Kang MK, Yang WI et al (2011) Clinical and echocardiographic predictors of outcomes in patients with apical hypertrophic cardiomyopathy. Am J Cardiol 108(11):1614–1619
Klarich KW, Attenhofer Jost CH, Binder J, Connolly HM, Scott CG, Freeman WK et al (2013) Risk of death in long-term follow-up of patients with apical hypertrophic cardiomyopathy. Am J Cardiol 111(12):1784–1791
Maron BJ, Maron MS, Semsarian C (2012) Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol 60(8):705–715
Teekakirikul P, Zhu W, Huang HC, Fung E (2019) Hypertrophic cardiomyopathy: an overview of genetics and management. Biomolecules 9(12):878
Mosqueira D, Smith JGW, Bhagwan JR, Denning C (2019) Modeling hypertrophic cardiomyopathy: mechanistic insights and pharmacological intervention. Trends Mol Med 25(9):775–790
Millat G, Bouvagnet P, Chevalier P, Dauphin C, Jouk PS, Da Costa A et al (2010) Prevalence and spectrum of mutations in a cohort of 192 unrelated patients with hypertrophic cardiomyopathy. Eur J Med Genet 53(5):261–267
Marian AJ, Braunwald E (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 121(7):749–770
Moro E, D’Angelo G, Nicolosi GL, Mimo R, Zanuttini D (1995) Long-term evaluation of patients with apical hypertrophic cardiomyopathy Correlation between quantitative echocardiographic assessment of apical hypertrophy and clinical-electrocardiographic findings. Eur Heart J 16(2):210–217
Monserrat L, Hermida-Prieto M, Fernandez X, Rodriguez I, Dumont C, Cazon L et al (2007) Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur Heart J 28(16):1953–1961
Arad M, Penas-Lado M, Monserrat L, Maron BJ, Sherrid M, Ho CY et al (2005) Gene mutations in apical hypertrophic cardiomyopathy. Circulation 112(18):2805–2811
Towe EC, Bos JM, Ommen SR, Gersh BJ, Ackerman MJ (2015) Genotype–phenotype correlations in apical variant hypertrophic cardiomyopathy. Congenit Heart Dis 10(3):E139–E145
Hughes RK, Knott KD, Malcolmson J, Augusto JB, Mohiddin SA, Kellman P et al (2020) Apical hypertrophic cardiomyopathy: the variant less known. J Am Heart Assoc 9(5):e015294
Flett AS, Maestrini V, Milliken D, Fontana M, Treibel TA, Harb R et al (2015) Diagnosis of apical hypertrophic cardiomyopathy: T-wave inversion and relative but not absolute apical left ventricular hypertrophy. Int J Cardiol 183:143–148
Wu B, Lu M, Zhang Y, Song B, Ling J, Huang J et al (2017) CMR assessment of the left ventricle apical morphology in subjects with unexplainable giant T-wave inversion and without apical wall thickness >/=15 mm. Eur Heart J Cardiovasc Imaging 18(2):186–194
Kim H, Park JH, Won KB, Yoon HJ, Park HS, Cho YK et al (2016) Significance of apical cavity obliteration in apical hypertrophic cardiomyopathy. Heart 102(15):1215–1220
Matsubara K, Nakamura T, Kuribayashi T, Azuma A, Nakagawa M (2003) Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J Am Coll Cardiol 42(2):288–295
Jan MF, Todaro MC, Oreto L, Tajik AJ (2016) Apical hypertrophic cardiomyopathy: present status. Int J Cardiol 222:745–759
Sherrid MV, Bernard S, Tripathi N, Patel Y, Modi V, Axel L et al (2023) Apical aneurysms and mid-left ventricular obstruction in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 16(5):591–605
Minami Y, Kajimoto K, Terajima Y, Yashiro B, Okayama D, Haruki S et al (2011) Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 57(23):2346–2355
Kim EK, Lee SC, Hwang JW, Chang SA, Park SJ, On YK et al (2016) Differences in apical and non-apical types of hypertrophic cardiomyopathy: a prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur Heart J Cardiovasc Imaging 17(6):678–686
Kao YC, Lee MF, Mao CT, Chen WS, Yang NI, Cherng WJ et al (2013) Differences of left ventricular systolic deformation in hypertensive patients with and without apical hypertrophic cardiomyopathy. Cardiovasc Ultrasound 11:40
Chen CC, Lei MH, Hsu YC, Chung SL, Sung YJ (2011) Apical hypertrophic cardiomyopathy: correlations between echocardiographic parameters, angiographic left ventricular morphology, and clinical outcomes. Clin Cardiol 34(4):233–238
Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS et al (2017) Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 69(7):761–773
Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RH et al (2015) Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol 65(18):1915–1928
Hughes RK, Augusto JB, Knott K, Davies R, Shiwani H, Seraphim A et al (2023) Apical ischemia is a universal feature of apical hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 16(3):e014907
Yang K, Song YY, Chen XY, Wang JX, Li L, Yin G et al (2020) Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur Heart J Cardiovasc Imaging 21(12):1341–1350
Yang K, Yu SQ, Chen XY, Song YY, Yang SJ, Cui C et al (2023) Apical aneurysm formation in apical hypertrophic cardiomyopathy: Pilot study with cardiac magnetic resonance. Int J Cardiol 371:480–485
Singam NSV, Stoddard MF (2019) The evolution of apical hypertrophic cardiomyopathy: development of mid-ventricular obstruction and apical aneurysm 11 years after initial diagnosis. Echocardiography 36(5):987–991
Binder J, Attenhofer Jost CH, Klarich KW, Connolly HM, Tajik AJ, Scott CG et al (2011) Apical hypertrophic cardiomyopathy: prevalence and correlates of apical outpouching. J Am Soc Echocardiogr 24(7):775–781
Paluszkiewicz J, Krasinska B, Milting H, Gummert J, Pyda M (2018) Apical hypertrophic cardiomyopathy: diagnosis, medical and surgical treatment. Kardiochir Torakochirurgia Pol 15(4):246–253
Yan L, Wang Z, Xu Z, Li Y, Tao Y, Fan C (2012) Two hundred eight patients with apical hypertrophic cardiomyopathy in China: clinical feature, prognosis, and comparison of pure and mixed forms. Clin Cardiol 35(2):101–106
Yusuf SW, Bathina JD, Banchs J, Mouhayar EN, Daher IN (2011) Apical hypertrophic cardiomyopathy. World J Cardiol 3(7):256–259
Eriksson MJ, Sonnenberg B, Woo A, Rakowski P, Parker TG, Wigle ED et al (2002) Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 39(4):638–645
Shin DG, Son JW, Park JY, Choi JW, Ryu SK (2015) Impact of coronary artery anatomy on clinical course and prognosis in apical hypertrophic cardiomyopathy: analysis of coronary angiography and computed tomography. Korean Circ J 45(1):38–43
Yamaguchi H, Ishimura T, Nishiyama S, Nagasaki F, Nakanishi S, Takatsu F et al (1979) Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol 44(3):401–412
Koga Y, Katoh A, Matsuyama K, Ikeda H, Hiyamuta K, Toshima H et al (1995) Disappearance of giant negative T waves in patients with the Japanese form of apical hypertrophy. J Am Coll Cardiol 26(7):1672–1678
Chen X, Dong JZ, Du X, Wu JH, Yu RH, Long DY et al (2018) Long-term outcome of catheter ablation for atrial fibrillation in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 29(7):951–957
Tower-Rader A, Kramer CM, Neubauer S, Nagueh SF, Desai MY (2020) Multimodality imaging in hypertrophic cardiomyopathy for risk stratification. Circ Cardiovasc Imaging 13(2):e009026
Nagueh SF, Phelan D, Abraham T, Armour A, Desai MY, Dragulescu A et al (2022) Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: an update from the american society of echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 35(6):533–569
Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D’Andrea A et al (2015) Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 16(3):280
Louie EK, Maron BJ (1987) Apical hypertrophic cardiomyopathy: clinical and two-dimensional echocardiographic assessment. Ann Intern Med 106(5):663–670
Webb JG, Sasson Z, Rakowski H, Liu P, Wigle ED (1990) Apical hypertrophic cardiomyopathy: clinical follow-up and diagnostic correlates. J Am Coll Cardiol 15(1):83–90
Huang G, Fadl SA, Sukhotski S, Matesan M (2020) Apical variant hypertrophic cardiomyopathy “multimodality imaging evaluation.” Int J Cardiovasc Imaging 36(3):553–561
Unlu S, Duchenne J, Mirea O, Pagourelias ED, Bezy S, Cvijic M et al (2020) Impact of apical foreshortening on deformation measurements: a report from the EACVI-ASE Strain Standardization Task Force. Eur Heart J Cardiovasc Imaging 21(3):337–343
Chang SA, Kim HK, Kim DH, Kim JC, Kim YJ, Kim HC et al (2010) Left ventricular twist mechanics in patients with apical hypertrophic cardiomyopathy: assessment with 2D speckle tracking echocardiography. Heart 96(1):49–55
Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL et al (2017) Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging 18(11):1205–1205af
Amano Y, Kitamura M, Takano H, Yanagisawa F, Tachi M, Suzuki Y et al (2018) Cardiac MR imaging of hypertrophic cardiomyopathy: techniques, findings, and clinical relevance. Magn Reson Med Sci 17(2):120–131
Kebed KY, Al Adham RI, Bishu K, Askew JW, Klarich KW, Araoz PA et al (2014) Evaluation of apical subtype of hypertrophic cardiomyopathy using cardiac magnetic resonance imaging with gadolinium enhancement. Am J Cardiol 114(5):777–782
Parisi R, Mirabella F, Secco GG, Fattori R (2014) Multimodality imaging in apical hypertrophic cardiomyopathy. World J Cardiol 6(9):916–923
To AC, Dhillon A, Desai MY (2011) Cardiac magnetic resonance in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 4(10):1123–1137
Narula J, Chandrashekhar Y, Ahmadi A, Abbara S, Berman DS, Blankstein R et al (2021) SCCT 2021 Expert Consensus Document on coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr 15(3):192–217
Williams TJ, Manghat NE, McKay-Ferguson A, Ring NJ, Morgan-Hughes GJ, Roobottom CA (2008) Cardiomyopathy: appearances on ECG-gated 64-detector row computed tomography. Clin Radiol 63(4):464–474
Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X et al (2008) 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 94(11):1386–1393
Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL (2007) A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 49(8):863–871
Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C et al (2023) 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J 44(37):3503–3626
Maron BJ (2018) Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 379(20):1977
Authors/Task Force M, McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A et al (2022) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 24(1):4–131
Shimada YJ, Passeri JJ, Baggish AL, O’Callaghan C, Lowry PA, Yannekis G et al (2013) Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail 1(6):480–487
Axelsson A, Iversen K, Vejlstrup N, Ho CY, Havndrup O, Kofoed KF et al (2016) Functional effects of losartan in hypertrophic cardiomyopathy-a randomised clinical trial. Heart 102(4):285–291
Axelsson A, Iversen K, Vejlstrup N, Ho C, Norsk J, Langhoff L et al (2015) Efficacy and safety of the angiotensin II receptor blocker losartan for hypertrophic cardiomyopathy: the INHERIT randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 3(2):123–131
Grillo MP, Erve JCL, Dick R, Driscoll JP, Haste N, Markova S et al (2019) In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica 49(6):718–733
Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM (2017) A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem 292(40):16571–16577
Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H et al (2018) Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci USA 115(35):E8143–E8152
Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM et al (2020) Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 75(21):2649–2660
Spertus JA, Fine JT, Elliott P, Ho CY, Olivotto I, Saberi S et al (2021) Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 397(10293):2467–2475
Maron MS, Rowin EJ, Wessler BS, Mooney PJ, Fatima A, Patel P et al (2019) Enhanced American College of Cardiology/American Heart Association Strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol 4(7):644–657
Tengiz I, Ercan E, Alioglu E, Turk UO (2006) Percutaneous septal ablation for left mid-ventricular obstructive hypertrophic cardiomyopathy: a case report. BMC Cardiovasc Disord 6:15
Inada K, Seiler J, Roberts-Thomson KC, Steven D, Rosman J, John RM et al (2011) Substrate characterization and catheter ablation for monomorphic ventricular tachycardia in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 22(1):41–48
Schaff HV, Brown ML, Dearani JA, Abel MD, Ommen SR, Sorajja P et al (2010) Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 139(3):634–640
Said SM, Schaff HV (2013) Surgical treatment of hypertrophic cardiomyopathy. Semin Thorac Cardiovasc Surg 25(4):300–309
Hang D, Schaff HV, Ommen SR, Dearani JA, Nishimura RA (2018) Combined transaortic and transapical approach to septal myectomy in patients with complex hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 155(5):2096–2102
Nguyen A, Schaff HV, Nishimura RA, Geske JB, Dearani JA, King KS et al (2019) Apical myectomy for patients with hypertrophic cardiomyopathy and advanced heart failure. J Thorac Cardiovasc Surg 159(1):145–152
Fang J, Liu Y, Zhu Y, Li R, Wang R, Wang DW et al (2023) First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 82(7):575–586
Kitaoka H, Doi Y, Casey SA, Hitomi N, Furuno T, Maron BJ (2003) Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol 92(10):1183–1186
Yang HS, Song JK, Song JM, Kang DH, Lee CW, Hong MK et al (2005) Comparison of the clinical features of apical hypertrophic cardiomyopathy versus asymmetric septal hypertrophy in Korea. Korean J Intern Med 20(2):111–115
Kim SH, Kim SO, Han S, Hwang KW, Lee CW, Nam GB et al (2013) Long-term comparison of apical versus asymmetric hypertrophic cardiomyopathy. Int Heart J 54(4):207–211
Klarich KW, AttenhoferJost CH, Binder J, Connolly HM, Scott CG, Freeman WK et al (2013) Risk of death in long-term follow-up of patients with apical hypertrophic cardiomyopathy. Am J Cardiol 111(12):1784–1791
Cai C, Duan FJ, Yang YJ, Guo XY, Liu YL, Liu YQ et al (2014) Comparison of the prevalence, clinical features, and long-term outcomes of midventricular hypertrophy vs apical phenotype in patients with hypertrophic cardiomyopathy. Can J Cardiol 30(4):441–447
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P et al (2020) 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 142(25):e533–e557
Li J, Zhang J, Shi Y, Sievert H, Taub CC, Bertog S et al (2023) Myocardial mechanics of percutaneous intramyocardial septal radiofrequency ablation. Heart 109(4):289–296
Subramanian M, Atreya AR, Yalagudri SD, Shekar PV, Saggu DK, Narasimhan C (2022) Catheter ablation for ventricular arrhythmias in hypertrophic cardiomyopathy. Card Electrophysiol Clin 14(4):693–699
Rastegar H, Boll G, Rowin EJ, Dolan N, Carroll C, Udelson JE et al (2017) Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: the Tufts experience. Ann Cardiothorac Surg 6(4):353–363
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Fang, J., Liu, Y. et al. Apical hypertrophic cardiomyopathy: pathophysiology, diagnosis and management. Clin Res Cardiol 113, 680–693 (2024). https://doi.org/10.1007/s00392-023-02328-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02328-8