Abstract
Introduction
Stroke prevention using oral anticoagulation (OAC) is the first management priority in atrial fibrillation (AF). Despite the importance of good therapy adherence, real-world adherence is still suboptimal. Patient education and adherence monitoring with new technologies are recommended. The main purpose of this sub-analysis of the AF-EduCare trial was to evaluate the effect of personalized follow-up strategies on adherence to OAC.
Methods
Regimen adherence was monitored by the electronic Medication Event Monitoring System cap at the start of the trial (M1) and after 12 months (M2), each for three months. Patients were part of one of three education groups (In-person, Online or App-based) or the standard care (SC) group. All are qualified for OAC therapy.
Results
A total of 768 patients were evaluated (11.8% SC vs. 86.8% any education group, mean age: 70.1 ± 7.9 years). Patients were taking non-vitamin K OAC (once daily 53.8%; twice daily 35.9%) or vitamin K antagonists (9.4%), equally distributed over the different study arms (p = 0.457). Mean therapy adherence was high (M1:93.8 ± 10.8%; M2:94.1 ± 10.1%). During both monitoring periods, the education group scored significantly higher than SC (M1:94.2 ± 10.0% vs. 91.3 ± 15.0%; p = 0.027; M2:94.4 ± 9.3% vs. 91.6 ± 14.0%; p = 0.006). More patients in the In-person and Online groups were able to keep or improve their adherence to > 90% compared to the SC.
Conclusion
Overall adherence to OAC in all study groups, even in SC, was very high, without attrition over time. Nevertheless, targeted education led to a small but significantly improved adherence compared to SC.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment with oral anticoagulation (OAC) is one of the main pillars in managing atrial fibrillation (AF), as the risk of stroke is five times higher in patients with AF compared to persons without AF [1]. About 90% of AF patients take OAC, either vitamin K antagonists (VKA) or non-vitamin K antagonist oral anticoagulants (NOAC), the latter being now the preferred strategy (in the absence of mechanical prosthetic heart valves or moderate to severe mitral stenosis) [2, 3] Four NOACs are commercially available: the twice-daily taken (BID) apixaban or dabigatran, and the once-daily taken (OD) rivaroxaban or edoxaban. For optimal efficacy of these NOACs because of their short anticoagulation effect, strict adherence to the recommended dose schedule is essential to reduce the risk for thrombo-embolic and bleeding events in comparison with the VKAs [4].
However, real-world adherence to NOAC therapy is not always optimal. Recent reviews and meta-analyses of Salmasi et al. and Ozaki et al. showed that about 30% of AF patients are non-adherent and mean adherence ranges between 57 and 82% [5, 6]. It is clear that despite the convenience for patients of NOACs which do not require laboratory testing but require strict adherence, efforts are needed to monitor adherence and prevent non-adherence.
Besides patient education, electronic monitoring of medication intake can be used as a support tool to monitor medication intake, as suggested by the European Society of Cardiology (ESC) AF Management Guidelines and the European Heart Rhythm Association (EHRA) 2021 Practical Guide on the use of NOACs in AF patients [3, 7, 8]. Such measurements allow self-regulation of the intake habits of patients, which can lead to actions to optimize adherence.
Our aim was to investigate the effect of different educational interventions on regimen adherence to OAC (both VKA and NOAC) in a contemporary Belgian AF population.
Methods
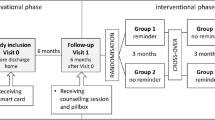
This study is part of a prospective, multicenter, randomized controlled trial executed in three Belgian hospitals (AF-EduCare study; NCT03707873 and AF-EduApp sub-study; NCT03788044) [9]. Within these trials the AF-EduCare approach was used, which is based on four aspects (discussed by Delesie et al.): (I) targeted education about AF and its treatment, (II) assessment of AF-related riks factors (RF) and informing patients on how to tackle these, (III) ensuring high therapy adherence with the use of electronic monitoring, and (IV) offering an easy way for patients to ask questions about AF and its management. The effect of different personalized AF education strategies (i.e., In-person, Online, or App-based education) on cardiovascular outcome of all types of AF patients compared to standard care (SC) is the primary outcome of the AF-EduCare trial. The AF-EduApp study was implemented as a sub-study in the AF-EduCare trial (Supplementary Fig. 1) with the AF-EduCare approach incorporated in the AF-EduApp application. Therapy adherence to OAC is the primary outcome of the AF-EduApp sub-study. With both trials having therapy adherence as outcome parameter (Supplementary Fig. 1), this paper focusses on the third pillar of the AF-EduCare approach (i.e., improving therapy adherence).
Study population and design
The whole study protocol is already published [9]. However, inclusion of patients and the different study groups will be explained briefly. All types of AF patients (i.e., > 18 years, able to speak and read Dutch, not cognitive impaired and with a life expectancy > 1 year), hospitalized at the cardiology ward or coming for an outpatient visit could be included in the AF-EduCare/AF-EduApp study. After patients agreed to participate, the patients were randomly assigned to one of three intervention groups (In-person, Online, App-driven education), or SC. Patients in the In-person group were seen in the hospital, where a research nurse gave them targeted education. Patients in the Online and App groups, however, had access to either an online education platform (i.e., with AF information and possibility to fill-out questionnaires) or the in-house developed AF-EduApp application (containing 6 main modules [10], explained in Supplementary Annex 1), respectively.
Intervention and data collection
One of the study interventions focuses on improving OAC therapy using an electronic Medication Event Monitoring System (MEMS, AARDEX Group, Liège, Belgium). Ambulatory or hospitalized AF patients randomized to the intervention groups, treated with a NOAC or VKA at baseline or initiated during the study, were monitored with the MEMS cap at the start of the study (Monitoring 1, M1) and again after one year (Monitoring 2, M2), each time for a period of three months. The app-based group was monitored during the whole 15 months; read-outs at the same time points were used as in the other groups. An LCD screen on the MEMS cap displays the number of openings of the medication bottle over 24 h, providing direct patient feedback about daily intake. Every bottle opening is stored as data in the MEMS cap build-in memory card, which was read out by the study team after the three-month monitoring period at the 3- and 15-months follow-up visits. Since VKA doses are variable and since dabigatran has to be stored in the original package to protect it from moisture, a proxy medication was chosen to measure adherence for these drugs, i.e., another oral drug of importance that the patient had to take OD or BID at the same time(s) of the day as VKA or dabigatran.
Regimen adherence was calculated as the number of days on which one bottle opening (in case of rivaroxaban, edoxaban, or a VKA-proxy medication) or two bottle openings (in case of apixaban or dabigatran-proxy medication) were registered, divided by the total number of monitored days and multiplied by 100. To correct the adherence estimates, bottle openings for refills or medication interruptions allowed by a physician were taken into account. To analyze the clinical effect of non-adherence between OD and BID, the number of unprotected days was calculated based on the simulation of Vrijens et al.: i.e., ≥ 1 or ≥ 3 consecutive missed doses for OD or BID respectively, or any extra doses over the dosing regimen in a time interval of 24 h, were considered as an ‘unprotected day’ [11].
Patients with a regimen adherence < 80% were categorized as “low adherence”, following the definition most used in the literature [6]. The “low adherence” patients received additional adherence telemonitoring for three months immediately after their regular adherence monitoring period. With telemonitoring, the data of the MEMS are transferred immediately by wireless transmission to an Internet server (through an NFC-compatible smartphone). Accordingly, telephone feedback was given in case of intake irregularities (i.e., for OD medication, more than one intake over 24 h or one missed dose; for BID medication, more than two intakes over 24 h or three consecutive missed doses). We have shown before that such telemonitoring with rapid feedback improves adherence [11, 12].
A small sample of AF patients randomized to the SC group was also monitored using a MEMS cap. This sample was kept as small as possible not to trigger too many patients in the control group to focus on their adherence on top of usual care, which would constitute a bias-by-measurement (Hawthorne effect) for the trial [13]. In addition, the MEMS cap in this group was not equipped with an LCD screen. Based on a standard deviation of regimen adherence in a control group of ± 20% [12] and a confidence interval of 5%, a minimum of 64 SC patients should be monitored with the MEMS by the end of the trial. Our goal was to measure OAC therapy adherence in a real-world setting with as little interference bias as possible. These SC patients were randomly selected by consecutive inclusion when MEMS caps without LCD screens were available and stratified to OAC medication.
To further assess the effect of using the MEMS on therapy adherence in the SC group, 24 additional SC patients received MEMS recording without an LCD screen after a prior period of three months during which pill count was performed: taking adherence (i.e., the proportion of prescribed doses taken) was compared between pill count and MEMS measurements.
Impact of COVID-19 on trial execution and on the methods described
The COVID-19 pandemic hit while the AF-EduCare trial and app sub-study were ongoing. During the period from March 2020 to March 2021, there were several periods with restrictions, such as lockdowns, where patients were only allowed to come to the hospital for urgent matters. Due to the restrictions, patients could not always comply with the study visits and used the medication bottle for longer periods than planned. An overview of the number of patients who had an M1 or M2 visit during this period is presented in Supplementary Table 1. To intervene with the proposed timeline of the patients as little as possible, outdoor drive-ins were organized. During these drive-ins, patients drove to the hospital parking lot; the MEMS was safely accepted and read out. After the reading, the patients were given a go if therapy adherence was higher than 80%, or the necessary equipment for adherence telemonitoring (MEMS with smartphone) was given if regimen adherence was < 80%.
Statistical analysis
Data were analyzed using IBM SPSS version 28.0 (IBM Corporation, Armonk, USA). Variables were described as numbers and percentages, mean ± standard deviation (SD), or median and interquartile range (IQR) as appropriate. Normal distribution was assessed using the Shapiro–Wilk test. For continuous variables, differences between two or more groups were compared using the Mann–Whitney U test or the Kruskal–Wallis test (non-parametric). The chi-squared test was used for categorical variables when appropriate. Bonferroni correction was used to correct for multiple comparisons after which p values < 0.05 were considered statistically significant.
Results
Demographics
A total of 768 patients of the AF-EduCare/AF-EduApp study were eligible for monitoring of therapy adherence (Fig. 1), of which 91 (11.8%) received standard care, and 677 (86.8%) received targeted education (i.e., 313 (40.8%) in-person, 224 (29.2%) online and 140 (18.2%) app-based education). No data of the MEMS was available for a total of 284 patients. The most frequent reason patients were excluded during both monitoring periods was refusal to use the medication bottle (114 of the 284 excluded patients; 40.1%) as patients claimed to continue their medication intake habits. Measurements of patients who used the medication bottle for less than 30 days for different reasons (e.g., technical issues, termination of medication…) were also excluded (10.6%).
The patients had a mean age of 70.1 ± 7.9 years, 69.7% were male, with a mean CHA2DS2-VASc score of 3.2 ± 1.6. A total of 72 (9.4%) patients were taking VKA, and 689 (89.7%) patients were on a NOAC. Another seven patients (0.9%) were not on any OAC at the start of the study but initiated OAC during the study, at which monitoring was also initiated (OAC initiated in first three months: 2 patients, OAC initiated after three months: 5 patients). The randomly selected sample of patients in the standard care group was well-matched with the intervention group (Table 1). In total, 614 patients were monitored successfully during M1, and 502 patients were monitored successfully during M2. A total of 18 patients only had a second period without a first measurement due to various reasons (i.e., no OAC at the start (n = 5), short period on OAC (n = 2), did not want to use MEMS (n = 7), < 30 days of use (n = 2), MEMS broken (n = 2)). Overall, 483 patients were followed during both monitoring periods.
Therapy adherence to OAC
During M1 (n = 614), overall regimen adherence was 93.8 ± 10.8% with a mean registration period of 100 ± 43.6 days. Patients in the intervention groups had a significantly higher regimen adherence than the SC group (94.2 ± 10.0% vs. 91.3 ± 15.0%; p = 0.029), driven by a significant higher therapy adherence of the Online group compared to SC (p = 0.030) (Fig. 2A). There was no significant difference between the different intervention groups (p = 0.148). Also, when looking at various adherence thresholds (i.e., 80%, 90%, and 95%), there was a significant difference between the four study groups (p = 0.049) (Fig. 2B). However, there were no significant differences when pairwise comparisons were performed between the four study groups. Visually, the app group seems to show an adherence more comparable to the SC group than to the other two intervention groups, but this could not be demonstrated statistically (Fig. 2C and D).
Due to various circumstances, fewer patients had an M2 (Fig. 1). A total of 502 patients showed an overall regimen adherence of 94.1 ± 10.1% (mean registration duration of 107.2 ± 49.6 days). Again, patients in the intervention groups combined had significantly higher adherence than patients in the standard care group (94.4 ± 9.3% vs. 91.6 ± 14.0%; p = 0.006) (Fig. 3A). This was mainly driven by a significantly higher score in the in-person group (95.4 ± 7.3%) compared to the standard care group (p = 0.015). Therapy adherence of the app group did not significantly differ from both other study groups (93.0 ± 10.7 vs. 94.8 ± 8.9; p = 0.560) or the SC (91.6 ± 14.0; p = 0.415). Also, the combined in-person and online adherence was significantly higher than SC (p = 0.009) (Fig. 3C). Comparing categories of therapy adherence showed a significant difference between the four study groups (p = 0.041) (Fig. 3B), but again without significant differences with the pairwise comparison of the four study groups. Compared to SC, the combined online and in-person groups showed a significant difference (p = 0.015). The app group did not significantly differ from the other two intervention groups (p = 0.114) or the SC (p = 1.00) (Fig. 3D).
Impact of telemonitoring
Of the 26 patients (out of 537 intervention patients, i.e., 4.8%) with a regimen adherence lower than 80% during M1, 24 patients completed a telemonitoring period of three months with daily follow-up and phone feedback by the study team if needed. This significantly increased their regimen adherence (from 60.0 ± 22.1% to 89.5 ± 12.3%; p < 0.001). Eighteen out of the 436 intervention patients (4.1%) had a regimen adherence lower than 80% during M2, of whom only eight patients completed a telemonitoring period, which significantly improved therapy adherence (from 51.4 ± 17.0% to 89.9 ± 9.7%; p = 0.012). The patients who did not start telemonitoring did not want to use the smartphone with NFC or did not want to use the MEMS again.
Impact of AF-EduCare approach on therapy adherence to OAC over time
A total of 483 patients fulfilled both monitoring periods. Overall, therapy adherence was preserved between the two monitoring periods (M1: 94.3 ± 9.8% vs. M2: 94.5 ± 9.1%; p = 0.538). In all groups separately, also in the SC, patients were able to sustain their therapy adherence over time (Fig. 4A). When making three categories of therapy adherence over time (i.e., patients who had a therapy adherence > 90% during both follow-up periods, patients with adherence < 90% during M1 and who improved above 90% at M2, or patients who did not improve > 90%), there were significant adherence differences between the study groups (p = 0.047) (Fig. 4B). Again, the app group seems to be more comparable with the SC and lower than the in-person and online combined. However, this could not be demonstrated statistically (p = 1.00 and p = 0.141, respectively). Only patients in the in-person and online combined scored better than patients in the SC group (p = 0.027). When looking at patients with therapy adherence < 90% during M1 (n = 120), Table 2 seems to suggest that education patients (especially in-person) improved more throughout the study than SC patients, although this did not reach statistical significance (p = 0.087).
Patients who were motivated to use the MEMS during two monitoring periods had a higher therapy adherence at M1 (94.3 ± 9.8%) compared to patients who only used it during M1 (92.0 ± 13.9; p = 0.060) (Table 3), with good preservation of this high adherence over time (M1: 94.3 ± 9.8% vs. 94.5 ± 9.1; p = 0.538; Table 3). When looking at the app group, only 37 of the 84 patients (44.0%) were motivated enough to use the MEMS during the whole study, as prescribed per protocol. These patients had a significantly higher mean therapy adherence (96.2 ± 5.3%) during M2 compared to the 47 patients who used the MEMS only from 12 to 15 months (92.5 ± 7.1%; p = 0.048). This difference was already emerging at M1, although not yet significant (96.3 ± 4.8% vs. 93.1 ± 8.0%; p = 0.067).
High therapy adherence in standard care
A group of 22 SC patients was evaluated by both pill count and MEMS monitoring. Eight had to be excluded because they did not fulfill the requirements for either the MEMS or pill count data set, and four more had to be excluded as they had forgotten to bring all their remaining OAC medication to the pill count appointments. In the remaining 10, there was a significant difference in taking adherence as measured by pill count or MEMS usage, 92.3 ± 6.8% vs. 98.3 ± 3.4%, respectively; p = 0.019.
Adherence of once vs. twice daily taken OAC and its potential therapeutic impact
During both monitoring periods, OD OAC regimen adherence was significantly higher compared to BID adherence (M1: OD 94.6 ± 9.9% vs. BID 92.3 ± 12.1%, p < 0.001; M2: OD 94.9 ± 9.8% vs. BID 92.5 ± 10.9%, p < 0.001). However, when looking at unprotected days based on the manuscript of Vrijens et al.[11], there was a trend of BID having significantly fewer unprotected days than OD (4.1 ± 9.0 vs. 5.5 ± 10.5 unprotected days, respectively, p = 0.050) at M1. This was confirmed during M2 with 3.1 ± 6.1 vs. 5.5 ± 10.2 unprotected days for BID and OD OAC regimens, respectively; p = 0.002.
Discussion
This report is the first large prospective study investigating adherence for all OACs using electronic monitoring. The results show that AF patients generally had a very high regimen adherence (94.3 ± 9.8% and 94.5 ± 9.1% at first, respectively, second measurement period, one year apart). This could be related to the fact that all patients consented to participate in a clinical trial, presumably preselecting more eligible patients. Nevertheless, educational intervention optimized adherence, but often not statistically significant when delivered via an app. In addition, we confirm prior findings that MEMS combined with tele-monitoring and immediate phone-based feedback leads to a very significant improvement in those with an initial adherence of < 80%. Finally, while regimen adherence is higher in OD than in BID, the projected steadiness of clinical protection may not be different or even better for BID regimens.
Therapy adherence to OAC
With the advent of NOACs, guidelines have stressed the importance of correct intake, i.e., regimen adherence. Different studies illustrate that this increased focus leads to improvements. A recently published study in a large cohort of more than 500.000 AF patients from European countries, newly started on NOAC, showed an increase in the proportion of those with a Medication Possession Ratio (MPR) ≥ 90% after one year from 62% in 2011 to 80% in 2017 [14]. Likewise, a study using prescription data of NOACs from Sweden (n = 5.254) and the Netherlands (n = 430) found that 86.6% and 87.2% of the patients, respectively, had an MPR > 90% [15]. A study in a Belgian cohort of 766 patients showed a median MPR of 95.2% (IQR = 87.8–99.7), although 31% had an MPR < 90% [16]. Another recently published large cohort study in Belgium (> 200.000 patients) based on prescription data showed that almost 90% of NOAC patients had a high proportion of days covered (PDC, > 90%), with a mean PDC after one year of 97.3% ± 5.8% in persistent users [17]. Nevertheless, MPR and PDC mostly overestimate therapy adherence compared to regimen adherence, as they do not consider the number of correct doses on each day. The very high adherence in our study, therefore, is reassuring. It is comparable to two other studies using electronic monitoring, which reported values of 91.9% and 93.8% using similar technology [12, 18]. It is clear that adherence to NOAC therapy has improved over the years in many countries. Apart from the study context, our high values may also reflect that Belgium is one of the leading countries in terms of guideline adherence for OAC treatment. This confirms studies in other therapeutic domains, in which Belgian patients showed a higher therapy adherence than other European countries [2, 19].
Impact of telemonitoring
Regardless of the overall high regimen adherence, about 4–5% of the patients were clearly sub-adherent (< 80%). Telemonitoring with feedback significantly improved their regimen adherence during both follow-up periods, indicating that this technique is an option for low-adherence patients. In a prior study by our group in which telemonitoring-based telephone feedback was given to all patients, regimen adherence increased from 93.8 to 96.8% (p = 0.002) [12]. Despite telemonitoring, mean adherence in the patients with < 80% adherence in this trial did not improve to > 90% (only to 89.5 ± 12.3% and 89.9 ± 9.7% during periods 1 and 2, respectively), i.e., the generally considered threshold for effective therapy to prevent adverse outcomes (e.g., stroke, major bleeding, myocardial infarction) [20]. Nevertheless, 16 of 24 and 6 of 8 individual patients improved beyond this threshold.
Impact of AF-EduCare approach on therapy adherence to OAC in general and over time
Patients in the intervention groups with targeted education scored significantly higher during both monitoring periods than the SC group. The higher adherence in the education groups might be related to the education itself and/or the use of MEMS with an LCD screen providing direct patient feedback. The high therapy adherence during the first monitoring period showed an early impact, which was maintained after one year when the second monitoring was performed.
Two other studies have evaluated the impact of education on adherence to NOAC therapy. In the AEGEAN study, 1228 AF patients, who were NOAC naïve and initiated on apixaban, were included at different European centers and randomized to SC or an education program (Edu) [18]. The education program consisted of an education booklet on AF and OAC treatment, access to reminder tools, and access to a virtual clinic that called the patients regularly. After 24 weeks, patients in the Edu group were re-randomized to further education or a secondary SC group for again 24 weeks. Adherence was measured using electronic monitoring as in our study, albeit with a different medication holder (Helping Hand, WestRock Switzerland Ltd., Sion, Switzerland). Mean regimen adherence in the Edu group was 91.9 ± 16.1% after 24 weeks and 90.4 ± 18.0% after 48 weeks, slightly lower than in our study. No significant difference (p > 0.07) was seen between the groups at 24 weeks (Edu: 91.9 ± 16.1%; SC: 91.6 ± 17.1%) or 48 weeks (Edu: 90.4 ± 18.0%; SC: 90.1 ± 18.6% secondary SC: 89.3 ± 18.1%). Another study investigated the effect of a pharmacist-led educational intervention on regimen adherence for NOAC [21]. A total of 301 patients were included in the SMAAP-AF study who received electronic monitoring (i.e., a card-type electronic device attached to the press-through package) to begin with an observational phase. Afterward, they were randomized to standard care and educational intervention. Despite a significant increase in therapy adherence in both groups, the mean change in medication adherence over time was not significantly different between the two groups (i.e., SC: 2.9% [7.5%] vs. education 3.4% [8.3%]). As in the AF-EduCare study, AEGEAN and SMAAP-AF patients in the SC group had very high regimen adherence (i.e., 91.6 ± 17.1% after 24 weeks and 90.1 ± 18.6% after 48 weeks in AEGEAN, and 94.5% and 92.9% in SMAAP-AF), making it challenging to demonstrate that education improves therapy adherence. Nevertheless, unlike AEGEAN and SMAAP-AF, our intervention groups scored significantly higher than the SC group during both monitoring periods. This may be related to the personalized and targeted education on AF in general and its management used in our study.
When comparing the different educational follow-up strategies, in-person education had the highest impact on therapy adherence, with 90.8% of patients having a therapy adherence > 90%. The app-driven educational follow-up strategy (i.e., education via an application and the possibility to install medication reminders in the application) scored lower compared to the other two education groups, with only 79.7% of patients with a therapy adherence > 90% (p = 0.114). The lower therapy adherence could be related to app fatigue during follow-up. This was indeed seen in the pilot study, with a significant decrease in app usage after one month [10]. Less app-driven education and feedback could lead to fewer patients taking their medication correctly compared to the in-person group who regularly saw the study nurse. Although the online group has a similar number of per-protocol in-person visits as the app group, the online group seemed to score better than the app group and similar to the in-person group. The findings may be confounded by the fact that more patients in the app group (67.3%) had their first monitoring period during COVID-19 peaks compared to the in-person (29.2%) and online (31.7%) groups. As a result, these patients had more telephone follow-ups instead of in-person visits, which could result in less adequate feedback and stimulation for good therapy adherence. Based on all the combined results, it seems important to have a minimum number of personal visits to successfully motivate patients to better adherence rates. It is also good to note that due to the educational follow-up, more than 10% of the patients improved their therapy adherence to > 90% after one-year follow-up.
A reason for the high regimen adherence in all trials could be using an electronic device, leading to an overestimation of adherence due to the awareness of being monitored (i.e., the Hawthorne effect [13]). This was confirmed by our data in a subset of SC patients which showed an overestimation of 6% when adherence was based on electronic monitoring vs. pill count. El Alili et al. (8%) also reported this in a recent review [22]. Other reasons for high therapy adherence could be related to an increased focus on integrated care and patient-centricity in general (i.e., the type of standard care in which the patient is a central figure and more involved in their care), selection bias of people that consented to participate in a clinical trial, or the high exclusion rate of 35.7% who did not want to use the MEMS.
Adherence of once vs. twice daily taken OAC and its potential therapeutic impact
Regimen adherence to OACs with an OD regimen was significantly higher (albeit minor) than those with a BID regimen during each monitoring period, which was also shown by several other studies [12, 17, 23]. The difference is small and should not preclude other adherence-improving measures (like education) to improve adherence for both OD and BID OACs. Paradoxically, OD NOACs may result in lower clinical protection, as was implicated by Vrijens et al., who simulated that missing one OD dose is equal to missing three consecutive BID doses [11]. The study of Desteghe et al. already showed fewer unprotected days (based on this definition) in BID regimens compared to OD [12]. In our study, patients on BID had significantly fewer unprotected days compared to OD regimen. These results show the important difference between regimen adherence and the clinical relevance of unprotected days. Further studies on clinical outcomes are needed to investigate this effect further.
Usability of MEMS
Despite the usability of the MEMS to monitor therapy adherence to OAC, various patients still do not want to use it due to multiple reasons. Of the 744 patients needing to be started with MEMS monitoring, only 484 (65.1%) completed both monitoring periods. Nevertheless, 615 patients (82.7%) used MEMS correctly for three months during the first monitoring period. Hence, the non-use during the second period may be more related to patient fatigue than inherent problems with the technology itself [24]. We have shown that patient motivation for MEMS relates to regimen adherence to OAC. Whether that is a causal or coincidental finding merits further study. In any case, MEMS proves to be a viable tool for monitoring and increasing patients' awareness of adherence and possible pitfalls.
Limitations
The MEMS is a good tool for monitoring therapy adherence. Nevertheless, it requires extra effort from the patients who, for example, use a medication pill box or another medication intake system, given that the medication to be monitored has to be put in the bottle. The high drop-out rate due to the use of the MEMS could also bias the high adherence rate in this study. In addition, using this MEMS somehow alarmed the patient for being observed, which could improve therapy adherence compared to regular medication taking. This makes it more difficult to objectively measure adherence in the standard care group which could affect the comparison of the standard care group with the intervention groups. With this study, it was shown that telemonitoring led to a significant improvement. However, this was performed in only a small sample of patients who had therapy adherence < 80%. It remains hard to assess the impact of COVID-19 on our study. Despite all mitigation efforts, it certainly reduced personal contact with the patients through which it may have affected findings.
Conclusions
Based on the results of this study, this Belgian AF population, in general, had already a very high regimen adherence to OAC intake. Nevertheless, an educational intervention further optimized adherence (albeit less so when delivered via an app with less in-person contact) and could prevent attrition of adherence. Monitoring therapy adherence is an additional educational tool to provide insights into the structural causes of non-adherence, while telemonitoring is particularly useful in AF patients with very low adherence (< 80%). The extent to which mHealth tools can replace in-person visits to maintain therapy adherence should be further investigated.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Abbreviations
- AF:
-
Atrial fibrillation
- BID:
-
Twice-daily taken
- EHRA:
-
European Heart Rhythm Association
- ESC:
-
European Society of Cardiology
- M1:
-
First monitoring period (baseline until 3 months)
- M2:
-
Second monitoring period (from 12 until 15 months)
- MEMS:
-
Medication Event Monitoring System
- MPR:
-
Medication Possession Ratio
- NOAC:
-
Non-vitamin K antagonist oral anticoagulant
- OAC:
-
Oral anticoagulation
- OD:
-
Once-daily taken
- RF:
-
Risk factor
- SC:
-
Standard care
- VKA:
-
Vitamin K antagonist
References
Friberg L, Rosenqvist M, Lindgren A, Terént A, Norrving B, Asplund K (2014) High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke 45(9):2599–2605
Cools F, Wollaert B, Vervoort G, Verstraete S, Voet J, Hermans K et al (2019) Treatment patterns in anticoagulant therapy in patients with newly diagnosed atrial fibrillation in Belgium: results from the GARFIELD-AF registry. Acta Cardiol 74(4):309–318
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al (2020) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 42(5):373–498
Rivera-Caravaca JM, Esteve-Pastor MA, Roldán V, Marín F, Lip GYH (2017) Non-vitamin K antagonist oral anticoagulants: impact of nonadherence and discontinuation. Expert Opin Drug Saf 16(9):1051–1062
Ozaki AF, Choi AS, Le QT, Ko DT, Han JK, Park SS et al (2020) Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 13(3):e005969
Salmasi S, Loewen PS, Tandun R, Andrade JG, De Vera MA (2020) Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open 10(4):e034778
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L et al (2018) The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39(16):1330–1393
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG et al (2021) 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 23(10):1612–1676
Delesie M, Knaepen L, Dendale P, Vijgen J, Ector J, Verbeeck J et al (2021) Effect of targeted education for atrial fibrillation patients: design of the EduCare-AF Study. Eur J Clin Invest 51(1):e13442
Knaepen L, Delesie M, Theunis R, Vijgen J, Dendale P, Desteghe L et al (2021) A new smartphone application for integrated transmural care of atrial fibrillation, AF-EduApp: usability and validation study. Digital Health 7:12
Vrijens B, Heidbuchel H (2015) Non-vitamin K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace. 17(4):514–23
Desteghe L, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J, Dendale P et al (2018) Telemonitoring-based feedback improves adherence to non-vitamin K antagonist oral anticoagulants intake in patients with atrial fibrillation. Eur Heart J 39(16):1394–1403
Wickström G, Bendix T (2000) The, “Hawthorne effect”—what did the original Hawthorne studies actually show? Scand J Work Environ Health 26(4):363–367
Komen JJ, Pottegård A, Mantel-Teeuwisse AK, Forslund T, Hjemdahl P, Wettermark B et al (2021) Persistence and adherence to non-vitamin K antagonist oral anticoagulant treatment in patients with atrial fibrillation across five Western European countries. Europace 23(11):1722–1730
Jacobs MS, Schouten JF, de Boer PT, Hoffmann M, Levin L, Postma MJ (2018) Secondary adherence to non-vitamin-K antagonist oral anticoagulants in patients with atrial fibrillation in Sweden and the Netherlands. Curr Med Res Opin 34(10):1839–1847
Capiau A, Mehuys E, Van Tongelen I, Christiaens T, De Sutter A, Steurbaut S et al (2020) Community pharmacy-based study of adherence to non-vitamin K antagonist oral anticoagulants. Heart 106(22):1740–1746
Grymonprez M, Capiau A, Steurbaut S, Mehuys E, Boussery K, De Backer TL et al (2022) Adherence and persistence to oral anticoagulants in patients with atrial fibrillation: a Belgian nationwide cohort study. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2022.994085
Montalescot G, Brotons C, Cosyns B, Crijns HJ, D’Angelo A, Drouet L et al (2020) Educational impact on apixaban adherence in atrial fibrillation (the AEGEAN STUDY): a randomized clinical trial. Am J Cardiovasc Drugs 20(1):61–71
Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I et al (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos Int 26(10):2479–2489
Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS et al (2020) The optimal drug adherence to maximize the efficacy and safety of non-vitamin K antagonist oral anticoagulant in real-world atrial fibrillation patients. Europace 22(4):547–557
Shiga T, Kimura T, Fukushima N, Yoshiyama Y, Iwade K, Mori F et al (2022) Effects of a pharmacist-led educational interventional program on electronic monitoring-assessed adherence to direct oral anticoagulants: a randomized, controlled trial in patients with nonvalvular atrial fibrillation. Clin Ther. https://doi.org/10.1016/j.clinthera.2022.09.011
El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M (2016) A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 82(1):268–279
McHorney CA, Crivera C, Laliberté F, Nelson WW, Germain G, Bookhart B et al (2015) Adherence to non-vitamin-K-antagonist oral anticoagulant medications based on the pharmacy quality alliance measure. Curr Med Res Opin 31(12):2167–2173
Shaw RJ, Steinberg DM, Bonnet J, Modarai F, George A, Cunningham T et al (2016) Mobile health devices: will patients actually use them? J Am Med Inform Assoc 23(3):462–466
Acknowledgements
The AF-EduCare study is a project supported by the Fund for Scientific Research, Flanders (T002917N). AARDEX Group, Liège, Belgium, partly supported the use of the electronic Medication Event Monitoring System (MEMS). The AF-EduApp study was supported by a BMS-Pfizer Alliance European Thrombosis Investigator Initiated Research Program (ERISTA) grant. This study is part of the Limburg Clinical Research Center, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. We would also like to thank the study nurses and AF nurse specialists of the involved hospitals for recruiting and following up on the patients in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.H. did receive personal lecture and consultancy fees from Biotronik and BMS-Pfizer Alliance. He received unconditional research grants through the University of Antwerp and/or the University of Hasselt from Bayer, Boehringer-Ingelheim, Bracco Imaging Europe, Abbott, Medtronic, Biotronik, Daicchi-Sankyo, BMS-Pfizer Alliance, and Boston-Scientific, all outside the scope of this work.
Ethical approval
This clinical study was approved by the leading Ethics Committee of UZA/UA and took into account the advice of the local ethics committees.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knaepen, L., Delesie, M., Vijgen, J. et al. Adherence to oral anticoagulation measured by electronic monitoring in a Belgian atrial fibrillation population. Clin Res Cardiol 112, 1812–1823 (2023). https://doi.org/10.1007/s00392-023-02261-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02261-w