Abstract
Background
The sodium-glucose co-transporter 2 inhibitor empagliflozin improves cardiovascular outcome in patients with type 2 diabetes mellitus (T2DM) and heart failure. Experimental studies suggest a direct cardiac effect of empagliflozin associated with an improvement in left ventricular diastolic function.
Methods
In the randomized, double-blind, two-armed, placebo-controlled, parallel group trial EmDia, patients with T2DM and elevated left ventricular E/E´ ratio were enrolled and randomized 1:1 to receive empagliflozin 10 mg/day versus placebo. The primary endpoint was the change of left ventricular E/E´ ratio after 12 weeks of intervention.
Results
A total of 144 patients with T2DM and an elevated left ventricular E/e´ ratio (age 68.9 ± 7.7 years; 14.1% women; E/e´ ratio 9.61[8.24/11.14], left ventricular ejection fraction 58.9% ± 5.6%). After 12 weeks of intervention, empagliflozin resulted in a significant higher decrease in the primary endpoint E/e´ ratio by − 1.18 ([95% confidence interval (CI) − 1.72/− 0.65]; P < 0.0001) compared with placebo. The beneficial effect of empagliflozin was consistent across all subgroups and also occurred in subjects with heart failure and preserved ejection fraction (n = 30). Additional effects of empagliflozin on body weight, HbA1c, uric acid, red blood cell count, hemoglobin, mean corpuscular hemoglobin, and hematocrit were detected (all P < 0.001). Approximately one-third of the reduction in E/e´ by empagliflozin could be explained by the variables examined.
Conclusions
Empagliflozin improves diastolic function in patients with T2DM and elevated end-diastolic pressure. Since the positive effects were consistent in patients with and without heart failure with preserved ejection fraction, the data add a mechanistic insight for the beneficial cardiovascular effect of empagliflozin.
Trial registration
Clinicaltrials.gov, unique identifier: NCT02932436.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is an epidemic disease affecting more than 460 million people worldwide [1]. In recent years, inhibitors of the sodium-dependent glucose co-transporter 2 (SGLT2) have received marketing approval as antidiabetic agents following positive cardiovascular outcome trials: in the EMPA-REG Outcome trial, the SGLT2 inhibitor empagliflozin reduced the risk of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke by 14% compared with placebo, mainly due to a strong reduction in cardiovascular death [2]. Analysis of the cumulative incidence demonstrated a separation between both groups that was detectable as early as two months after initiation of therapy. In a secondary analysis, empagliflozin was associated with a 35% reduction of hospitalization for heart failure, also observed immediately after treatment initiation, suggesting a very early effect on the failing heart [3]. Together with evidence from several other cardiovascular outcome trials, accumulating evidence to a paradigm shift in antidiabetic therapy. Clinical trials are have recently been completed reporting beneficial efficacy and safety of empagliflozin compared to placebo in patients with heart failure with and without diabetes mellitus [4, 5].
The specific mechanisms mediating the beneficial effects of empagliflozin on cardiovascular outcome remain controversial [6]. Previous studies have suggested that the effect on the heart may be related to an improvement in left ventricular diastolic function [7, 8]. However, comprehensive evidence from randomized studies in humans that investigate the effects of empagliflozin on cardiac function along with humoral cardiac, metabolic and hematological biomarkers is currently lacking.
The EmDia trial was designed to evaluate the effect of empagliflozin compared to placebo on left ventricular diastolic function in subjects with type 2 diabetes mellitus and elevated left ventricular end-diastolic pressure in a randomized, double-blind controlled clinical trial combined with comprehensive clinical and molecular phenotyping.
Methods
Trial design
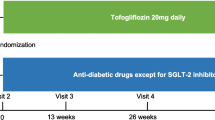
The EmDia trial is a randomized, double-blind, two-armed, placebo-controlled, parallel group, investigator-initiated study of phase IV. The University Medical Center of the Johannes Gutenberg-University Mainz conducted the single-center trial as study sponsor. All study documents were approved by the local ethics committee and the data protection officer prior to study initiation. All study participants provided informed written consent, and study procedures have been performed in line with the principles outlined in the Declaration of Helsinki and the recommendations for Good Clinical Practice. The trial was registered at clinicaltrials.gov with the unique identifier: NCT02932436 (EudraCT number: 2016-001264-11). The rationale and design of the trial have been described in detail recently [9].
Patient enrolment and randomization
The main inclusion criteria of the EmDia trial were: (i) age from 18 to 84 years, (ii) diagnosis of type 2 diabetes mellitus with stable glucose-lowering background therapy and/or dietary treatment for at least 12 weeks, (iii) HbA1c ≥ 6.5% and ≤ 10.0% in subjects on antidiabetic background therapy or HbA1c ≥ 6.5% and ≤ 9.0% for drug-naïve subjects with dietary treatment, and (iv) prevalent left ventricular diastolic dysfunction defined as left ventricular lateral E/E´ ratio ≥ 8 in transthoracic echocardiography. Main exclusion criteria were impaired renal function, defined as estimated glomerular filtration rate (eGFR [10]) < 45 ml/min/1.73 m2 of body-surface-area or end-stage renal failure or dialysis or uncontrolled hyperglycemia with a glucose level > 240 mg/dl (> 13.3 mmol/L) after overnight fast.
Patients who met all inclusion criteria and none of the exclusion criteria were randomized 1:1 to the intervention or control group at the baseline visit. Block-randomization including sex-stratification was performed by an independent institution (Interdisciplinary Center for Clinical Trials (IZKS), Mainz, Germany). During the 12-week trial period after randomization, patients received empagliflozin at a dose of 10 mg per day or an identical placebo in addition to the concomitant medication.
Trial procedures
At the dedicated study center, patients received a highly standardized 5-h clinical and medical technical examination from October 2016 to June 2020. Trained and certified medical assistants performed all procedures according to standard operating procedures. Comprehensive phenotyping was performed identically at visit 1 (baseline visit) and after 12 weeks of the intervention (visit 3). In addition, patients received a follow-up visit one week after randomization (visit 2).
During the visit at the study center, information on current medication (according to Anatomical Therapeutic Chemical (ATC) classification system), cardiovascular risk factors and comorbidities (e.g., atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, myocardial infarction, peripheral artery disease stroke, and venous thromboembolism) was collected through physical examination, computer-assisted interviews, anthropometric and blood pressure measurements, as well as laboratory analysis (see Supplemental Appendix for detailed information). In addition to medical-technical examinations, which were mainly focused on the cardiovascular system, blood and urine samples were taken and subsequently stored at − 80 °C for biobanking.
Transthoracic echocardiography was conducted using an iE33 echocardiography system with an S5-1 sector array transducer (Royal Philips Electronics, Amsterdam, The Netherlands). Measurements of cardiac structure and function were taken according to current guideline recommendations [11]. All datasets were digitally transferred to a server with an integrated multimodal image management system (Xcelera, Royal Philips Electronics, Amsterdam, The Netherlands) and reviewed by an experienced board-certified cardiologist in a blinded manner.
Study endpoints
The primary endpoint of the EmDia trial was defined as the change of E/E´ ratio after 12 weeks of intervention. In this report, the following secondary and tertiary endpoints have been explored: left ventricular ejection fraction, left ventricular end-diastolic volume, left ventricular mass index, humoral biomarkers of cardiovascular disease (i.e., troponin, N-terminal pro brain natriuretic peptide (NT-proBNP), C-reactive protein), blood count (i.e., red blood cell count, leukocyte count, platelet count, hemoglobin, hematocrit, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, mean platelet volume), vital signs (i.e., systolic and diastolic blood pressure, heart rate), and metabolism (i.e., HbA1c, body mass index, uric acid, and fatty liver index).
Statistical analysis
All randomized subjects who received at least one dose of trial treatment and had at least one available post-baseline assessment of the primary analysis variable were included in the intention-to-treat (ITT) population (primary analysis sample). Continuous variables are presented by mean and standard deviation for normal distributions and by median and interquartile range for skewed distributions. Discrete variables are described by relative and absolute frequencies.
To account for potential differences between groups and to increase statistical power, it was pre-specified that the analysis of study endpoints would be performed by linear regression analysis with the study endpoint as the dependent variable and empagliflozin 10 mg/day versus placebo as predictor, adjusting for age, sex, and baseline value of each outcome parameter. The respective estimates provide the difference of the change scores by groups. To assess the robustness of the potential effect of empagliflozin on the primary study endpoint, sensitivity analyses were performed in the per-protocol sample and in clinically relevant subgroups: stratified by preserved and reduced left ventricular ejection fraction (≥ 55% vs. < 55%), NT-proBNP within and outside the reference range (< 125 pg/ml vs. ≥ 125 pg/ml), presence of congestive heart failure, left ventricular hypertrophy, and obesity, but also level of uric acid, eGFR, and HbA1c. Finally, mediation analysis using linear regression was performed to quantify the contribution of changes in selected biomarkers to the effect of empagliflozin on the E/e´ ratio after 12 weeks of intervention. In this study, a P-value < 0.05 was considered as statistically significant. Statistical analyses were performed using the R software package (Version 4.0.3).
Results
Cohort characteristics at baseline
Out of a total of 301 individuals screened, N = 144 subjects were enrolled and randomized in the EmDia trial. The analysis sample consisted of N = 142 patients with at least one follow-up assessment of left ventricular E/´ ratio. The mean age of the study cohort was 68.9 ± 7.7 years with 14.1% female subjects. The clinical characteristics of the analysis sample stratified by treatment group are displayed in Table 1. No significant differences were observed between the intervention group (empagliflozin 10 mg/day) and the control group (placebo) with regard to clinical profile including traditional cardiovascular risk factors and comorbidities. The most frequently recorded comorbidities were coronary artery disease (40.2%), followed by atrial fibrillation (30.2%) and chronic heart failure (21.1%). Among individuals with chronic heart failure, the predominant HF phenotype were HF with preserved ejection fraction (HFpEF; n = 21) and HF with mildly reduced ejection fraction (HFmrEF; n = 9); no subject suffered from HF with reduced ejection fraction (HFrEF) at baseline. HbA1c levels at baseline did not differ between groups with 7.4% (interquartile range (IQR) 7.0%/8.2%) for the placebo group and 7.3% (IQR 7.0%/7.7%) for the empagliflozin group (P for difference = 0.30). Regarding the primary outcome measure E/e´ ratio, a significant difference was observed at baseline: 9.06 (IQR 8.06/10.30) in patients receiving placebo compared to 9.90 (IQR 8.40/11.90) in patients receiving empagliflozin 10 mg/day (P = 0.031). Left ventricular ejection fraction (placebo: 57.9% ± 5.7% vs. empagliflozin: 59.2% ± 5.5%, P = 0.19) and NT-proBNP (placebo: 128.9 pg/ml (IQR 53.2 pg/ml /352.5 pg/ml) vs. empagliflozin: 141.7 pg/ml (IQR 59.2 pg/ml /268.2 pg/ml), P = 0.96) did not differ significantly between strata.
Effect of empagliflozin compared to placebo on left ventricular diastolic function
The E/e´ ratio decreased from baseline [9.90 (IQR 8.40/11.90)] through week 1 ]9.49 (IQR 8.14/11.18)] and week 12 (9.12 (IQR 7.51/10.80)) of intervention in individuals receiving empagliflozin 10 mg/day, while no change in E/e´ ratio was observed in individuals receiving placebo. The analysis of the primary study endpoint, i.e., the change in E/e´ ratio after 12 weeks of intervention, in a linear regression analysis with E/e´ ratio at 12 weeks as dependent variable and adjustment for the covariates age, sex, and E/e´ ratio at baseline, demonstrated that empagliflozin led to a significant decrease in E/e´ ratio by − 1.18 (95% confidence interval (CI) − 1.72 to − 0.65; P < 0.0001) after 12 weeks of intervention compared with placebo (see Fig. 1). This result was confirmed in the analysis of the primary study endpoint in the per-protocol sample (β-estimate: − 1.17 (95% CI − 1.73 to − 0.62), P < 0.0001) and also in sensitivity analysis with additional adjustment for arterial hypertension (β-estimate: − 1.15 (95% CI − 1.69/− 0.61, P < 0.0001). When analyzing the components of E/e´ ratio separately a trend for a beneficial effect of empagliflozin on both E (β-estimate: − 0.79, 95% CI − 8.34 to 0.143; P = 0.06) and e´ (β-estimate: 0.45, 95% CI − 0.06 to 0.95; P = 0.088) was observed. In the placebo group, there was no significant decrease of E/e´ ratio after 12 weeks of intervention (P = 0.53).
Pre-specified analysis of the effect of empagliflozin 10 mg/day versus placebo on the primary study endpoint E/e´ ratio and selected echocardiographic endpoints after 12 weeks of treatment. The figure displays the estimates of linear regression models with adjustment for age, sex, and baseline value of E/e´ ratio. The dependent variable is left ventricular E/e´ ratio after 12 weeks of intervention (Pattern A) and left ventricular ejection fraction, left ventricular end-diastolic volume and left ventricular mass index (Pattern B), respectively. The beta-estimate is given for the effect of empagliflozin (10 mg/day) versus placebo with accompanying 95% confidence interval. In addition, mean crude values (with standard deviation) of the endpoint measures are provided in the first line. Complete information on the primary outcome measure, i.e., left ventricular E/e´ ratio, was available for on N = 136 individuals (empagliflozin group: N = 67; placebo group: N = 69). CI confidence interval, LVEDV left ventricular end-diastolic volume, LVEF left ventricular ejection fraction, LVMI left ventricular mass index
Analysis of the short-term effect of empagliflozin on diastolic function after 1 week of intervention indicated a decrease in E/e´ ratio by empagliflozin compared with placebo (β-estimate: − 0.52 (95% CI − 1.10 to 0.06), although this result did not pass the threshold of statistical significance (P = 0.081).
Additional study endpoints
To comprehensively evaluate the effects of empagliflozin 10 mg/day compared with placebo in individuals with type 2 diabetes mellitus, further endpoints were analyzed. First, the change of left ventricular ejection fraction, left ventricular mass index, and left-ventricular end-diastolic volume throughout the study was assessed in crude analysis. Subsequently, a linear regression analysis with adjustment for age, sex, and baseline value of the respective biomarker confirmed the absence of an impact of empagliflozin on left ventricular systolic function and left ventricular hypertrophy after 1 and 12 weeks of treatment, respectively (see Table 2). For NT-proBNP levels, a short-term effect was detected after 1 week of intervention (β-estimate: − 0.23 (95% CI − 0.30 to − 0.06), P = 0.009), which was not seen after 12 weeks (β-estimate: − 0.01 95% CI − 0.195 to 0.175), P = 0.91). A similar result was registered for the effect of empagliflozin on systolic blood pressure (β-estimateafter 1 week: − 4.53 (95% CI − 8.13 to − 0.92), P = 0.015) and diastolic blood pressure (β-estimateafter 1 week: -3.05 (95% CI − 5.05 to − 1.05), P = 0.0034). With regard to measures of vascular function (i.e., carotid-femoral pulse wave velocity, augmentation index, arterial stiffness index and reflection index), no statistical effect of empagliflozin compared to placebo was detectable. The ankle–brachial index was not affected by empagliflozin after 12 weeks of intervention compared to placebo (beta-estimate: − 0.0158, 95% CI − 0.0578 to 0.0261; P = 0.46). Furthermore, no changes were observed for relative wall thickness, troponin or heart rate under treatment.
Due to the known impact of empagliflozin on body weight, further metabolic biomarkers were explored. Multivariable regression analysis revealed a significant effect of empagliflozin 10 mg/day versus placebo on body mass index (β-estimate: − 0.71 (95% CI − 0.99 to − 0.44; P < 0.0001), HbA1c (β-estimate: − 0.43 (95% CI − 0.62 to − 0.23), P < 0.0001), and uric acid (β-estimate: − 0.66 (95% CI − 1.01 to − 2.96), P = 0.0005) after 12 weeks of intervention. No significant effect of empagliflozin was found with respect to C-reactive protein and fatty liver index levels.
Last, the effect of empagliflozin on hematological biomarkers was analyzed. No significant effect of empagliflozin on leukocyte and platelet count was found, whereas a highly significant increase in red blood cell count was detected (β-estimate: 0.29 (95% CI 0.20–0.38), P < 0.0001). In addition, mean corpuscular hemoglobin concentration was reduced by empagliflozin (β-estimate: − 0.36 (95% CI − 0.61 to − 0.12), P = 0.005), whereas hematocrit (β-estimate: 2.91 (95% CI 2.09–3.73; P < 0.0001) and level of hemoglobin (β-estimate: 0.82 (95% CI 0.55–1.09), P < 0.0001) were increased by 12 weeks of therapy with empagliflozin.
Sensitivity and mediation analyses for the change in left ventricular diastolic function
In a next step, sensitivity analysis in clinically relevant subgroups has been carried out (Fig. 2). Of clinical relevance, stratified analysis by preserved versus reduced left ventricular ejection fraction, NT-proBNP in- and outside the reference range, presence of congestive heart failure, of obesity or left ventricular hypertrophy, levels of eGFR, HbA1c and uric acid demonstrated consistency and robustness of the effect of empagliflozin 10 mg/day compared with placebo on left ventricular E/e´ ratio across subgroups. For the subgroup with congestive heart failure, a mean LVEF of 54.6% ± 5.6% (placebo group) and 56.2% ± 7.0% (intervention group) was documented at baseline examination (lowest LVEF: 43.2%), indicating the predominant prevalence of heart failure with preserved ejection fraction in the cohort.
Effect of empagliflozin 10 mg/day vs. placebo on the primary study endpoint after 12 weeks of intervention in clinically-relevant subgroups. Beta-estimates for the effect of empagliflozin 10 mg/day compared to placebo on E/e´ ratio after 12 weeks of intervention are calculated by linear regression model with adjustment for age, sex, and baseline value of E/e´ ratio stratified by subgroups. Subgroups of eGFR and HbA1c were analyzed as predefined in the study protocol. HbA1c and uric acid were stratified by median, whereas LV ejection fraction was stratified by 55% according to distribution and NT-proBNP was stratified according to its use in the diagnosis of heart failure according to current guidelines. The squares with horizontal lines represent beta-estimates and corresponding confidence intervals. BNP brain natriuretic peptide, CI confidence interval, eGFR estimated glomerular filtration rate, LV left ventricular
To decipher the contribution of the systemic effects of empagliflozin on the change in left ventricular diastolic function, a mediation analysis for the effect of 12-week empagliflozin on E/E´ ratio was carried out. As illustrated in Table 3, the contribution of the following parameters to the change in E/E' ratio was investigated: body mass index, systolic and diastolic blood pressure, eGFR, HbA1c, NT-proBNP, uric acid, hematocrit, and hemoglobin. The strongest effects on the change in left ventricular diastolic function were determined by the effect of empagliflozin on hemoglobin, hematocrit, and glomerular filtration rate. Overall, approximately a third of the reduction of E/e´ ratio by empagliflozin was found to be explained via the impact of empagliflozin on all variables explored in the analysis (β–estimatefully adjusted: − 0.77 (95% CI − 1.52 to − 0.02), P = 0.046).
Discussion
The present study investigated the effects of the SGLT2 inhibitor empagliflozin in patients with diastolic dysfunction and type 2 diabetes mellitus. The results indicate a significant improvement in diastolic function as measured by left ventricular E/e´ ratio after 12 weeks of therapy with empagliflozin 10 mg/day compared to placebo as an adjunct to standard therapy, which was consistent across subgroups. The change in E/e´ ratio was accompanied by significant effects of empagliflozin on metabolic and hematologic biomarkers in this cohort with predominantly preserved left ventricular ejection fraction, which in turn explain approximately one-third of the beneficial effect of the drug on left ventricular diastolic function.
The results of the EmDia trial add to the growing body of evidence supporting a positive effect of empagliflozin on cardiovascular health [2]. Although these data provide clear evidence of an effect on cardiovascular disease, the underlying mechanisms still need to be defined. With regard to echocardiographic studies, a recent meta-analysis that pooled data from very small study samples revealed that empagliflozin may have a beneficial effect on E/e´ ratio [12]. Interestingly, data supporting a positive impact of SGLT2 inhibitors on the E/e´ ratio are more consistent for individuals at risk of developing heart failure (i.e., heart failure at American Heart Association (AHA) stage A/B) [13,14,15] as compared with individuals with symptomatic heart failure of AHA stage C [16,17,18]. Recent data from a subgroup analysis of the Empire HF trial showed that empagliflozin reduced left ventricular and left atrial volumes in patients with heart failure and reduced ejection fraction, which is consistent with findings from another randomized trial in non-diabetic subjects with heart failure and reduced ejection fraction [19, 20]. In contrast, the EMPA-HEART CardioLink-6 trial did not show a positive impact of empagliflozin on left ventricular diastolic function in individuals with diabetes mellitus and coronary artery disease [21]. The improvement in diastolic function found in the EmDia trial is also supported by recent data establishing a reduction in pulmonary pressures measured with an implanted pulmonary artery pressure sensor by empagliflozin compared to placebo [22]. Given the known beneficial effect of mineralocorticoid receptor antagonists on E/e´ ratio[23] and subsequent reduction in hospitalization for heart failure[24], the reduction in E/e´ ratio observed in the EmDia trial is of specific interest in the context of the beneficial effect of empagliflozin in patients with heart failure and preserved ejection fraction with and without diabetes mellitus [25].
The transient reduction in systolic and diastolic blood pressure after one week of therapy supports the hypothesis that part, and in particular the early, cardiovascular effects of empagliflozin may be mediated via an improvement in ventricular loading through a reduction in afterload that is likely secondary to the diuretic effects of the drug [26]. In the present study results, the improvement in ventricular loading was reflected by the short-term decrease in NTproBNP and the diuretic effect of the drug was mirrored by the increase in hematocrit, which was also observed in other trials such as the EMPA-HEART CardioLink-6 study [13]. In addition, effects of empagliflozin on hematological and metabolic biomarkers were encountered in the present study: The impact of empagliflozin on red blood cells found in the EmDia trial is likely to be explained by the earlier reported SGLT2 inhibitor-mediated increase in erythropoietin production, a change in red blood cell morphology and iron utilization [27]. The reduction in plasmatic levels of uric acid under treatment is of clinical relevance given abundant evidence on the prognostic value of uric acid [28].
The improvement in diastolic function found in the EmDia trial provides new insights relevant in context of the results of the recently published EMPEROR-PRESERVED clinical trial investigating the efficacy and safety of empagliflozin in individuals with heart failure and preserved ejection fraction (defined by a left ventricular ejection fraction > 40%) independent of the presence of diabetes mellitus [5]. Since no interaction for improvement in diastolic function was found with the presence of congestive heart failure with LVEF > 40% in the EmDia trial, it seems likely that empagliflozin will also improve diastolic dysfunction in patients with heart failure and preserved ejection fraction irrespective of the presence of diabetes mellitus. Further research is needed, however, to clarify whether empagliflozin emerges as novel treatment approach in chronic heart failure independent of the heart failure phenotype.
Strengths and limitations
The major strength of the present study is the well-phenotyped cohort, which was studied in a dedicated study center by trained staff in a highly standardized setting minimizing variability of data assessment at high accuracy and reproducibility. However, several limitations should be noted when interpreting the study results. The assessment of diastolic function was limited to left ventricular E/e´ ratio, which precluded consideration of other markers for cardiac function that have been reported to predict cardiovascular outcome, such as left atrial volume index or cardiac strain [29]. Since left ventricular E/e´ ratio is known to predict cardiovascular outcome [30], the demonstrated effects of empagliflozin on diastolic function are likely to be clinically relevant. As patients were recruited in a clinically stable condition, findings cannot be translated to the setting of acute decompensated heart failure. Due to limited sample size in the subsample of patients with HF, analysis stratified by HF phenotypes could not be performed. Finally, only one dosage of empagliflozin was investigated. However, a dose-dependent effect on diastolic function seems unlikely, as the effects of empagliflozin on cardiovascular outcome did not differ substantially between doses [31].
Conclusions
The results of the present EmDia trial demonstrated that empagliflozin 10 mg/day improved diastolic function in patients with type 2 diabetes mellitus and elevated left ventricular end-diastolic pressure within 12 weeks of treatment. The beneficial effect of empagliflozin was consistent across all subgroups and also occurred in subjects with heart failure and preserved ejection fraction, supporting the positive effect of empagliflozin reported in the literature regarding the treatment of patients with heart failure and including patients with heart failure with preserved ejection fraction. Because the identified positive hemodynamic, metabolic, and hematological effects of empagliflozin explain only part of its effect on cardiac function, future studies will be important to identify to what extent this mechanism contributes to improved clinical outcome in subjects with heart failure.
Data availability
This project constitutes a major scientific effort with high methodological standards and detailed guidelines for analysis and publication. Data are not made available for the scientific community outside the established and controlled workflows and algorithms. To meet the general idea of verification and reproducibility of scientific findings, we offer access to data at the local database in accordance with the ethics vote on request (contact: Prof. Dr. Philipp Wild (PI), philipp.wild@unimedizin-mainz.de).
References
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V et al (2020) 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41(2):255–323
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128
Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A et al (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 37(19):1526–1534
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383(15):1413–1424
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385(16):1451–1461
Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61(10):2108–2117
Pabel S, Wagner S, Bollenberg H, Bengel P, Kovács Á, Schach C et al (2018) Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail 20(12):1690–1700
Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A et al (2021) Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res 117(2):495–507
Jünger C, Prochaska JH, Gori T, Schulz A, Binder H, Daiber A et al (2022) Rationale and design of the effects of EMpagliflozin on left ventricular DIAstolic function in diabetes (EmDia) study. J Cardiovasc Med (Hagerstown) 23(3):191–197
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39e14
Yu YW, Zhao XM, Wang YH, Zhou Q, Huang Y, Zhai M et al (2021) Effect of sodium-glucose cotransporter 2 inhibitors on cardiac structure and function in type 2 diabetes mellitus patients with or without chronic heart failure: a meta-analysis. Cardiovasc Diabetol 20(1):25
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21):1995–2008
Shim CY, Seo J, Cho I, Lee CJ, Cho IJ, Lhagvasuren P et al (2021) Randomized, controlled trial to evaluate the effect of dapagliflozin on left ventricular diastolic function in patients with type 2 diabetes mellitus: the IDDIA trial. Circulation 143(5):510–512
Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC (2020) A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J 41(36):3421–3432
Tanaka H, Hirata KI (2018) Potential impact of SGLT2 inhibitors on left ventricular diastolic function in patients with diabetes mellitus. Heart Fail Rev 23(3):439–444
Ejiri K, Miyoshi T, Kihara H, Hata Y, Nagano T, Takaishi A et al (2020) Effect of luseogliflozin on heart failure with preserved ejection fraction in patients with diabetes mellitus. J Am Heart Assoc 9(16):e015103
Carbone S, Billingsley HE, Canada JM, Bressi E, Rotelli B, Kadariya D et al (2020) The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: the CANA-HF study. Diabetes Metab Res Rev 36(8):e3335
Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbæk L et al (2021) Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire HF randomized clinical trial. JAMA Cardiol 6(7):836–840
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S et al (2021) Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 77(3):243–255
Rai A, Connelly KA, Verma S, Mazer CD, Teoh H, Ng MY et al (2022) Empagliflozin does not affect left ventricular diastolic function in patients with type 2 diabetes mellitus and coronary artery disease: insight from the EMPA-HEART CardioLink-6 randomized clinical trial. Acta Diabetol 59(4):575–578
Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F et al (2021) Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from EMpagliflozin Evaluation By MeasuRing ImpAct on HemodynamiCs in PatiEnts with Heart Failure (EMBRACE-HF) Trial. Circulation 143:1673–1686
Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W et al (2013) Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 309(8):781–791
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B et al (2014) Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370(15):1383–1392
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385(16):1451–1461
Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C et al (2020) Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 142(11):1028–1039
Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A et al (2020) Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 141(8):704–707
Filippatos GS, Ahmed MI, Gladden JD, Mujib M, Aban IB, Love TE et al (2011) Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. Eur Heart J 32(6):712–720
Tröbs SO, Prochaska JH, Schwuchow-Thonke S, Schulz A, Müller F, Heidorn MW et al (2021) Association of global longitudinal strain with clinical status and mortality in patients with chronic heart failure. JAMA Cardiol. 6:448–456
Benfari G, Miller WL, Antoine C, Rossi A, Lin G, Oh JK et al (2019) Diastolic determinants of excess mortality in heart failure with reduced ejection fraction. JACC Heart Fail 7(9):808–817
Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A et al (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J 37(19):1526–1534
Acknowledgements
We would like to thank all study participants of the EmDia trial for their participation in this clinical study. Our thanks go to the clinical staff of the study center, the Interdisciplinary Center for Clinical Trials Mainz (IZKS) for trial support (i.e., Dr. Kai Kronfeld, Dr. Silke Warnke, Christoph Medler, Dr. Jana Topsch, Anja Powaska, and Dr. Christian Ruckes) and all medical colleagues (i.e., Dr. Ernst Hauser, Dr. Christoph Fleckenstein, Dr. Judith Prochaska, Dr. Madeleine Busch, Wolfgang Reeh, Dr. Michael Drexler, Dr. Susanne Thiel, Dr. Konstantin Katsaros, Dr. Armin Schütz, Dr. Julia Weinmann-Menke, and Dr. Matthias M. Weber) contributing to the successful implementation and conduct of this project.
Funding
Open Access funding enabled and organized by Projekt DEAL. The EmDia trial is an academic, GCP sponsor-investigator clinical trial funded with support of Boehringer Ingelheim. Dr. Wild and Dr. Prochaska are funded by the Federal Ministry of Education and Research (BMBF 01EO1503). Dr. Gori, Dr. Münzel and Dr. Wild are principal investigators of the German Center for Cardiovascular Research (DZHK). Dr. Wild is principal investigator of the DIASyM research core (BMBF 161L0217A).
Author information
Authors and Affiliations
Contributions
JHP contributed to study design, collection of data, data analysis, and drafted and revised the manuscript. CJ contributed to study design, supported data analysis and revised the manuscript for important intellectual content. AS contributed to study design, performed statistical analysis, contributed to the interpretation of data and revised the manuscript for important intellectual content. NA contributed to data collection, revised the manuscript and provided critical intellectual input. FM revised the manuscript and provided critical intellectual input. MWH revised the manuscript and provided critical intellectual input. RB revised the manuscript and provided critical intellectual input. DZ contributed to study organization and conduct, revised the manuscript, and provided critical intellectual input. TK contributed to data collection, revised the manuscript, and provided critical intellectual input. SOT contributed to data collection, revised the manuscript, and provided critical intellectual input. KJL contributed to study design and data collection, revised the manuscript, and provided critical intellectual input. A.D. contributed to study design, data collection, revised the manuscript, and provided critical intellectual input. HB contributed to study design, supported statistical analysis, revised the manuscript and provided critical intellectual input. SJS revised the manuscript and provided critical intellectual input. TG contributed to study design, data collection, revised the manuscript and provided critical intellectual input. T.M. contributed to study oversight, revised the manuscript and provided critical intellectual input. PSW acquired funding, contributed to study design, data analysis, and interpretation of data, provided study oversight and performed critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Prochaska has received honoraria for lectures from Boehringer Ingelheim and Bayer AG outside the topic of the present study. Dr. Wild has received research funding outside the topic of the present study from Boehringer Ingelheim, Sanofi-Aventis, Bayer Healthcare, Daiichi Sankyo Europe, and Novartis, and received outside the topic of the present study honoraria for lectures or consulting from Boehringer Ingelheim, Bayer HealthCare, Evonik, AstraZeneca and Sanofi-Aventis. Dr. Tröbs has received lecture fees for Philips AG outside the submitted work. Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapies, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Edwards, Eidos, Eisai, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Sanofi, Shifamed, Tenax, and United Therapeutics.
Collaborators of the study
Dr. Jürgen H. Prochaska, Dr. Andreas Schulz, Dr. Natalie Arnold, Dr. Felix Müller, Dr. Marc William Heidorn, Rieke Baumkötter, Dr. Daniela Zahn, Dr. Thomas Koeck, Dr. Sven-Oliver Tröbs, Dr. Karl J. Lackner, Dr. Andreas Daiber, Dr. Harald Binder, Dr. Sanjiv J. Shah, Dr. Tommaso Gori, Dr. Thomas Münzel, Dr. Philipp S. Wild, Dr. Steffen Rapp; Interdisciplinary Center for Clinical Trials (IZKS) Mainz (i.e., Dr. Silke Warnke, Dr. Kai Kronfeld, Dr. Christian Ruckes, Christoph Medler).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prochaska, J.H., Jünger, C., Schulz, A. et al. Effects of empagliflozin on left ventricular diastolic function in addition to usual care in individuals with type 2 diabetes mellitus—results from the randomized, double-blind, placebo-controlled EmDia trial. Clin Res Cardiol 112, 911–922 (2023). https://doi.org/10.1007/s00392-023-02164-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02164-w