Abstract
Background
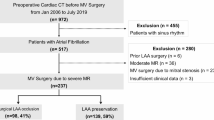
Patients undergoing left atrial appendage (LAA) occlusion (LAAO) are multi-morbid, including mitral valve disease (MVD) which is associated with anatomic changes of the left atrium (LA). This study aims to identify how atrial myopathy in MVD influences outcomes in LAAO.
Methods
Atrial myopathy in MVD was defined as LA diameter > 45 mm (♀) and > 48 mm (♂) and existing MVD or history of surgical/interventional treatment. Patients were compared with controls from the prospective, multicentre LAArge registry of LAAO.
Results
A total of 528 patients (52 MVD, 476 no-MVD) were included. The MVD group was significantly more likely to be older (78.2 years vs 75.9 years, p = 0.036) and female (59.6% vs 37.8%, p = 0.002). Altered LA anatomy was observed in MVD with significantly larger LA diameter (53 mm vs. 48 mm, p < 0.001) and LAA Ostia [at 135° 23.0 mm (20.5, 26.0) vs 20.0 mm (18.0, 23.0), p = 0.002]. Implant success was high with 96.2% and 97.9%, respectively, without differences in severe complications (7.7% vs 4.6%, p = 0.31). One-year mortality (17.8% vs 11.5%, p = 0.19) and a combined outcome of death, stroke, and systemic embolism (20.3% vs 12.4%, p = 0.13) were not different. Independent predictors of the combined outcome were peripheral artery disease (HR 2.41, 95% CI 1.46–3.98, p < 0.001) and chronic kidney disease (HR 3.46, 95% CI 2.02–5.93, p < 0.001) but not MVD and atrial myopathy.
Conclusion
Patients with MVD present with altered LA anatomy with increased LA and LAA diameter. However, procedural success and safety in LAAO are not compromised. One-year mortality is numerically higher in patients with MVD but driven by comorbidities.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia affecting more than 33 million people worldwide in 2010 [1]. AF is associated with an increase in stroke risk and mortality and its prevalence is rising [2]. While vitamin K antagonists such as Warfarin and non-vitamin K oral anticoagulants (NOAC) reduce stroke and mortality in AF, they substantially increase the risk for major bleedings [3]. In AF patients with a high risk for stroke and high risk for bleeding and/or contraindication to OAC, left atrial appendage occlusion (LAAO) may address both issues [4]. The five-year outcome data of the randomized controlled trials PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL-AF (Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) support a non-inferiority of LAAO to OAC with a significant decrease in hemorrhagic stroke [5]. However, comorbidities such as heart failure and chronic kidney disease impair clinical outcomes in patients with AF [6]. In fact, in patients undergoing transcatheter edge-to-edge repair (TEER), AF is associated with more bleeding, HF hospitalization, and mortality [7]. The mitral valve is of particular interest in LAAO because of the anatomical proximity and the effects on left atrial anatomy. Left atrial size is a predictor of cardiac death in heart failure before [8].
To our knowledge, this remains a field with no to little evidence, even though in a single-center study of 122 TEER patients, around 50% would also qualify for LAAO [9]. Currently, the first studies are evaluating a combined approach of both procedures even when no data on outcomes on LAAO in patients with mitral disease is available [10].
The German LAArge registry is an independently financed prospective, non-randomized registry of patients undergoing LAAO.
In this study, we aim to investigate the influence atrial myopathy due to mitral valve disease may have on in-hospital and long-term safety outcomes in patients after LAAO.
Methods
Data collection and LAArge registry
The non-profit organization “Institut für Herzinfarktforschung” (IHF, Ludwigshafen, Germany) manage and oversee the multicenter German left atrial appendage occlusion registry (LAArge). Thirty-eight centers participated in this prospective, non-randomized study. No funding from industry sources was used for the project. From all enrolled patients, written informed consent was obtained. The privacy measures and data collection have been described previously [11]. Using a web-based electronic case report form, baseline characteristics, procedural data as well as in-hospital data were collected and checked for plausibility. All transmitted data were encrypted and stored on servers maintained by IHF. Echocardiographic follow-up was documented at the standard follow-up at each participating site after LAAO, usually 3–6 months. The IHF conducted the 1-year follow-up by reports from the implanting center and via a standardized phone interview. The study was carried out according to the declaration of Helsinki and approved by the ethics committee of the State Chamber of Medicine in Rhineland-Palatinate, Germany.
Mitral valve disease definition and procedural methods
Mitral valve disease (MVD) was defined as pre-existing mitral regurgitation and/or TEER or surgical MV repair (SMVR) combined with a left atrial (LA) diameter of > 45 mm in women and > 48 mm in men. The reasoning was to include any irregular mitral valve anatomy, due to pathological MR or surgically or percutaneously treated MV. In addition, grading of mitral regurgitation after TEER becomes increasingly difficult. Patients were either allocated to the MVD group or the no-MVD group if the criteria were met. The detailed procedural methods have been described previously [11]. Patients were screened and enrolled following current guidelines and best medical practice [12]. Generally, AF patients with significant stroke risk and contraindication to anticoagulation were included. Device selection and pre-procedural imaging were left to the operator’s preference. The antithrombotic regime and procedural protocol were conducted according to the implanting center. Transesophageal echocardiography (TEE) was carried out to exclude patients with intracardiac thrombus and define anatomy for technical feasibility. Procedures were carried out in light sedation using propofol or general anesthesia.
Outcomes
In-hospital data included serious adverse events such as device embolization, peri-device leak, bleeding, stroke, and groin complications among others. Predefined safety outcomes are given in supplemental Table 1. Implantation success and adverse device events were defined as given in the Munich consensus document [13]. The primary outcome was a combined outcome of death, stroke, and systemic embolism after a year. Adverse events were collected for in-hospital events and follow-up events. Routine TEE follow-up was carried out but not mandatory to report as many implanting centers may have referred the patients to local centers for TEE follow-up.
Statistical analysis
Normally distributed continuous data are given as means ± of the standard deviation (SD), otherwise shown as medians with interquartile ranges (25th and 75th percentiles). Categorical data are presented in relative percentage and absolute values. Fisher’s exact test was used for rates of in-hospital complications. Statistical differences between both groups were compared using either a Chi-square test or the Mann–Whitney–Wilcoxon test. The 12-month event rates of death, the composite outcome of death and stroke, and a composite of death, stroke, and systemic embolism were calculated by the Kaplan–Meier method. The outcomes were compared between age groups using the log-rank test. Hazard ratios (HR) with 95% confidence intervals (CI) were estimated using Cox regression without adjustment. All statistical comparisons were two-sided, and p values < 0.05 were considered statistically significant. Analyses were performed using the Statistical Analysis System (SAS, Version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
A total of 555 patients were included in this study. The MVD cohort included 52 Patients and the no-MVD cohort 476 patients (Table 1). The MVD group was significantly more likely to be older (78.2 years ± 7.0 vs 75.9 years ± 8.0, p = 0.036) and female (59.6% vs 37.8%, p = 0.002). In the MVD cohort, 5.8% had a history of TEER and 11.5% had a history of SMVR. Cardiomyopathy (21.2% vs. 5.9%, p < 0.001) and chronic kidney disease (59.6% vs 36.8%, p = 0.001) were significantly higher in the MVD group. CHA2DS2-VASc (5.1 ± 1.5 vs. 4.5 ± 1.5, p = 0.009) and HAS-BLED-Score (4.4 ± 1.1 vs. 3.8 ± 1.1, p = 0.003) were significantly higher in MVD patients compared with the no-MVD group.
Left atrial appendage anatomy
Data for the left atrial appendage anatomy are presented in Table 2. The MVD cohort presented with significantly larger LA diameter (53 mm vs. 48 mm, p < 0.001) as well as larger LAA ostia (at 135° 23.0 mm (20.5, 26.0) vs 20.0 mm (18.0, 23.0), p = 0.002). There were no significant differences in LA sludge or LAA thrombus formation. The chicken wing morphology occurred the most in both groups (34.6% vs 46.6%, p = 0.10), followed by the windsock (13.5% vs 16.2%, p = 0.61) and cauliflower (17.3% vs 15.5%, p = 0.74) morphology and less often by the cactus (5.8% vs 9.9%, p = 0.33) morphology. Patients with MVD were more likely to have an atypical (classification not applicable) LAA morphology (28.8% vs 11.7%, p < 0.001).
Procedural data
Implant success was high in both cohorts with 96.2% and 97.9%, respectively. There was a significant difference in subpar device position in the MVD group [3.8% (2/52) vs 0.2% (1/476), p < 0.001] compared with the no-MVD cohort. No difference in device selection was reported. The Watchman device was the most common device (50% and 42.0%), followed by the Amplatzer Cardiac Plug (19.2% and 29.8%) and the Amplatzer Amulet (25.0% and 25.6%). Device embolization occurred in 1/52 procedures in the MVD cohort and 6/476 cases in the no-MVD cohort (p = 0.69). Peri-device leak and left–right shunt occurred in both groups without significant differences. No peri-device leaks > 5 mm were observed in either cohort (Table 3).
In-hospital safety data
The data for in-hospital safety are presented in Table 4. The MVD cohort had a longer hospital stay after the procedure compared with the no-MVD cohort [3 days (2, 6) vs 2 days (2, 3), p = 0.001]. There were no MACCE (death, stroke, or myocardial infarction) in the MVD cohort and 3 events in the no-MVD cohort. Other severe complications occurred in the MVD group with 5.8% and with 2.9% in the no-MVD cohort (p = 0.23). These included pericardial effusion with need for interventional drainage (3.58% vs 2.10%, p = 0.33), AV-Fistula (1.9% vs. 0.84%, p = 0.11), and severe bleedings (3.8% vs. 0.84%, p = 0.11) without significant differences between both groups. Moreover, moderate complications (13.5% vs 9.9%, p = 0.47) and overall complications (19.2% vs 12.8%, p = 0.20) were comparable between both cohorts.
Follow-up safety data
The Follow-up data are shown in Table 5. The follow-up rate was high in both groups with 92.3% in the MVD cohort and 98.7% in the no-MVD cohort. Device embolization was comparable in both groups (1.9% vs. 2.3%, p = 0.86). There were no differences in major complications including stroke, myocardial infarction, and moderate or severe bleeding. Follow-up echocardiography data were obtained in 42.3% and 31.9% of the patient at a mean follow-up duration of 163 and 97 days. No differences were observed in LA thrombi (4.5% vs 6.0%, p = 1.00) or overall peri-device leak (27.3% vs 16.6%, p = 0.24). Leaks over 5 mm were not observed in either cohort. One-year mortality was numerically higher in the MVD cohort (17.8% vs 11.5%, p = 0.19) as well as a combined outcome of death, stroke, and systemic embolism (20.3% vs 12.4%, p = 0.13) without reaching statistical significance (Fig. 1). Significant predictors of the combined outcome after adjusting were peripheral artery disease (HR 2.41, 95% CI 1.46–3.98, p < 0.001) and chronic kidney disease (HR 3.46, 95% CI 2.02–5.93, p < 0.001) but not the presence or history of MVD (Table 6). Further data on safety outcomes (Table S1) and antithrombotic therapy (Table S2) are provided in the supplement.
Discussion
Main findings
Patients with atrial myopathy and mitral valve disease referred for LAAO have more comorbidities than patients without a history of mitral valve disease. Even though larger LA and LAA orifices are observed, procedure success is high and comparable to patients without MVD. Cardiovascular events after one year are more common in patients with mitral valve disease but are driven by comorbidities.
Clinical characteristics and observed events
In patients with mitral valve disease, the occurrence of new-onset atrial fibrillation is high, reaching over 48% at a 10-year follow-up, and is associated with increased cardiac mobility [14]. In a more recent cohort of 2425 patients with degenerative mitral regurgitation, AF was associated with increased overall mortality [15]. In our study, MVD in a cohort of patients with atrial fibrillation is associated with numerically higher mortality. Both conditions are expected to rise with an aging population and increasing comorbidity.
We did not find that the presence of MVD and atrial myopathy is a predictor of cardiovascular events after LAAO. In line with previous data, comorbidities such as chronic kidney disease are stronger factors for mortality in AF [16]. These results are an important safety signal for patients with MVD as LAAO can be conducted in these patients without additional risk to the basic risk from comorbidities.
Another shared trait of both conditions is atrial myopathy with an increased size of both LA and LAA. In fact, in a prospective imaging study using both computed tomography and echocardiography, the most significant determinants of LA enlargement were AF and mitral regurgitation [17]. In our study, atypical morphology of the LAA was observed at a higher rate in those with MVD and atrial myopathy. LAA remodeling has been described in patients with persistent AF [18]. While this has not been reported in MVD, a higher volume load may lead to further remodeling.
However, it must be noted that atrial myopathy is a complex trait that is reflected in electrocardiograms, magnetic resonance imaging, strain imaging, and invasive electrophysiologic studies [19]. In the absence of this data, we chose the combination of LA enlargement and mitral valve disease in atrial fibrillation based on a study that reported that MR in HFpEF reflected LA myopathy even in the absence of AF [20]. Further studies have shown that the combination of mitral valve disease, atrial fibrillation, and left atrial enlargement is highly associated with LA myopathy [21].
Yet, implant success was high in both groups without a significant difference (96.2% vs. 97.9%). The complex anatomy and larger LAA observed with MVD and atrial myopathy are not associated with longer procedures or enhanced support with general anesthesia. While we observed a higher incidence of subpar device position with MVD, event rates are small and should be carefully considered. Device embolization and peri-device leak > 5 mm were rare in both groups and comparable, suggesting that operators adjusted for the different anatomy in patients with mitral valve disease. Postoperative hospitalization was longer in patients with MVD undergoing LAAO which reflects the higher burden of comorbidities such as chronic kidney disease (59.6% vs 36.8%), cardiomyopathy (21.2% vs 5.9%), and anemia (34.6% vs 21.6%).
Due to the proximity of the mitral valve to the orifice of the LAA, there is a concern that higher blood flow or regurgitant jet may impair endothelialization of the LAAO device once implanted [22]. Theoretically, a higher incidence of device-related thrombi (DRT) would be the result.
To our knowledge, there is no data on outcomes of patients with mitral valve disease after LAAO. Recently, a single-center case series of 122 AF patients undergoing TEER reported that around 50% would qualify for LAAO [9]. However, the study considered patients with a CHA2DS2-VASc score ≥ 3 and no contraindication to OAC as potential candidates. The recent European Society of Cardiology (ESC) guidelines on AF recommend LAAO only in patients with contraindication to OAC [4]. In our cohort, no differences in LA thrombi were observed; however, this may be due to low numbers of patients with MVD and even lower with complete echocardiographic follow-up data.
In addition, the impact of MVD on DRT is difficult to assess since larger LAA size itself is a strong predictor. For instance, in an analysis of 1739 patients from PREVAIL-AF, PROTECT-AF, and the continued registries, a 6% higher risk of DRT per mm LAA diameter increase (OR 1.06 per mm increase; 95% CI 1.01–1.12; p = 0.019) was observed [23]. A larger LAA diameter was also a predictor of DRT in the European EWOLUTION registry [24].
Strengths and limitations
There are some caveats to be considered. No standardized process for patient selection, implanting procedure, or postprocedural management. Due to the observational study design, confounding factors cannot be excluded. Additionally, we were limited by the available data in the registry and had to rely on diameter for LA and LAA sizing without more sensitive volumetric values such as left atrial volume index. While our definition of patients with AF, MVD, and enlarged LA sizes probably reflects atrial myopathy, we cannot report sophisticated data such as magnetic resonance imaging or data from electroanatomic mapping.
Echocardiographic follow-up data are insufficient for conclusive incidences of DRT and peri-device leak. Adverse events were reported by the implanting center and maybe therefore unreliable. Also, this was a purely interventional study without any data on rhythm or rate control. Therefore, no association regarding AF treatment can be described in our registry. We also have limited data on the temporal relation of events, for instance, whether pericardial effusion was a result of transseptal puncture or device release.
Our study has some unique strengths as this is an industry-independent study under real-life conditions. This is also the very first work to systematically report the outcomes of patients with atrial myopathy due to mitral valve disease in LAAO. We can also report the 1-year mortality of this cohort. Additionally, this study uses different devices and employs a standardized 1-year follow-up independent of the implanting centers.
Conclusion
Patients with MVD undergoing LAAO show atrial myopathy with larger LA diameter and LAA orifices. However, procedural success is not impaired and safety outcomes are comparable to patients without MVD. No differences in DRT or peri-device leak were observed and 1-year mortality is largely driven by comorbidities that must be addressed in management. Left atrial enlargement and MVD were not independent predictors of cardiovascular events.
Abbreviations
- AF:
-
Atrial fibrillation
- DRT:
-
Device-related thrombus
- LA:
-
Left atrium
- LAA:
-
Left atrial appendage
- LAAO:
-
Left atrial appendage occlusion
- MR:
-
Mitral regurgitation
- MVD:
-
Mitral valve disease
- NOAC:
-
Non-vitamin K oral anticoagulants
- OAC:
-
Oral anticoagulants
- SMVR:
-
Surgical mitral valve repair
- TEER:
-
Transcatheter edge-to-edge repair
References
Chugh SS et al (2014) Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129:837–847. https://doi.org/10.1161/CIRCULATIONAHA.113.005119
Schnabel RB et al (2015) 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 386:154–162. https://doi.org/10.1016/s0140-6736(14)61774-8
Piccini JP et al (2014) Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J 35:1873–1880. https://doi.org/10.1093/eurheartj/ehu083
Hindricks G et al (2020) ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa612
Reddy Vivek Y et al (2017) 5-Year outcomes after left atrial appendage closure. J Am Coll Cardiol 70:2964–2975. https://doi.org/10.1016/j.jacc.2017.10.021
Magnussen C et al (2017) Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 136:1588–1597. https://doi.org/10.1161/CIRCULATIONAHA.117.028981
Gertz ZM et al (2021) Implications of atrial fibrillation on the mechanisms of mitral regurgitation and response to MitraClip in the COAPT Trial. Circ Cardiovasc Interv 14:e010300. https://doi.org/10.1161/CIRCINTERVENTIONS.120.010300
Modena MG et al (1997) Left atrial size is the major predictor of cardiac death and overall clinical outcome in patients with dilated cardiomyopathy: a long-term follow-up study. Clin Cardiol 20:553–560
Lane CE, Eleid MF, Holmes DR Jr (2019) An under-recognized high-risk atrial fibrillation population: analyzing transcatheter mitral valve repair patients for left atrial appendage closure device application. Catheter Cardiovasc Interv 94:274–279. https://doi.org/10.1002/ccd.28220
D’Amico G et al (2021) Combined procedure of percutaneous mitral valve repair and left atrial appendage occlusion: a multicenter study. Cardiovasc Interv 14:590–592
Fastner C et al (2021) Left atrial appendage closure in patients with chronic kidney disease: results from the German multicentre LAARGE registry. Clin Res Cardiol 110:12–20. https://doi.org/10.1007/s00392-020-01638-5
Glikson M et al (2019) EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. EP Europace 22:184–184. https://doi.org/10.1093/europace/euz258
Tzikas A et al (2016) Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. EP Europace 19:4–15. https://doi.org/10.1093/europace/euw141
Grigioni F et al (2002) Atrial fibrillation complicating the course of degenerative mitral regurgitation. J Am Coll Cardiol 40:84–92. https://doi.org/10.1016/S0735-1097(02)01922-8
Grigioni F et al (2019) Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol 73:264–274. https://doi.org/10.1016/j.jacc.2018.10.067
Banerjee A et al (2013) Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire valley atrial fibrillation project. J Am Coll Cardiol 61:2079–2087. https://doi.org/10.1016/j.jacc.2013.02.035
Messika-Zeitoun D et al (2007) Left atrial remodelling in mitral regurgitation—methodologic approach, physiological determinants, and outcome implications: a prospective quantitative Doppler-echocardiographic and electron beam-computed tomographic study. Eur Heart J 28:1773–1781. https://doi.org/10.1093/eurheartj/ehm199
Kishima H et al (2016) Morphologic remodeling of left atrial appendage in patients with atrial fibrillation. Heart Rhythm 13:1823–1828. https://doi.org/10.1016/j.hrthm.2016.06.009
Shen MJ, Arora R, Jalife J (2019) Atrial myopathy. JACC Basic Transl Sci 4:640–654
Tamargo M et al (2020) Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail 22:489–498
Cameli M et al (2012) Left atrial speckle tracking analysis in patients with mitral insufficiency and history of paroxysmal atrial fibrillation. Int J Cardiovasc Imaging 28:1663–1670. https://doi.org/10.1007/s10554-011-9987-y
Sharma SP, Singh D, Nakamura D, Gopinathannair R, Lakkireddy D (2019) Incomplete endothelialization of WatchmanTM device: predictors and implications from two cases. J Atrial Fibrill 11:6
Dukkipati SR et al (2018) Device-related thrombus after left atrial appendage closure. Circulation 138:874–885. https://doi.org/10.1161/CIRCULATIONAHA.118.035090
Sedaghat A et al (2021) Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device—insights from the EWOLUTION real world registry. Catheter Cardiovasc Interv 97:E1019–E1024. https://doi.org/10.1002/ccd.29458
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was funded by an unrestricted grant from the foundation ‘Stiftung Institut für Herzinfarktforschung Ludwigshafen’ (Ludwigshafen, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MH, JS unrestricted grant from Boston Scientific; EL reports grants and personal fees from Abbott Vascular. Outside the submitted work EL received personal fees from Abiomed, Astra Zeneca, Bayer, Edwards Lifesciences, New Valve Technology and Novartis. Other authors report no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kany, S., Skibowski, J., Müller, CH. et al. Association of atrial myopathy in mitral valve disease on safety outcomes in left atrial appendage closure. Clin Res Cardiol 112, 824–833 (2023). https://doi.org/10.1007/s00392-022-02151-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02151-7