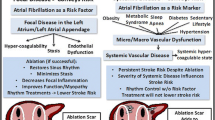
Abstract
Background
First-line ablation for atrial fibrillation (AF) reduces the risk of recurrent atrial arrhythmias compared to medical therapy. However, the prognostic benefit of early AF ablation remains undetermined. Herein, we aimed to evaluate the effects of early AF ablation compared to medical therapy.
Methods
Using data from phase II/III of the GLORIA-AF registry, we studied patients who were consecutively enrolled with newly diagnosed AF (< 3 months before baseline visit) and an increased risk of stroke (CHA2DS2–VASc ≥ 1). At baseline visit, 445 (1.7%) patients were treated with early AF ablation and 25,518 (98.3%) with medical therapy. Outcomes of interest were the composite outcome of all-cause death, stroke and major bleeding, and pre-specified outcomes of all-cause death, cardiovascular (CV) death, non-CV death, stroke and major bleeding.
Results
A total of 25,963 patients (11733 [45.2%] females; median age 71 [IQR 64–78] years; 17424 [67.1%] taking non-vitamin K antagonist oral anticoagulants [NOACs]) were included. Over a follow-up period of 3.0 (IQR 2.3–3.1) years, after adjustment for confounders, early AF ablation was associated with a significant reduction in the composite outcome of all-cause death, stroke and major bleeding (HR 0.50 [95% CI 0.30–0.85]) and all-cause death (HR 0.45 [95% CI 0.23–0.91]). There were no statistical differences between the groups in terms of CV death, non-CV death, stroke and major bleeding. Similar results were obtained in a propensity-score matched analysis of patients with comparable baseline variables.
Conclusions
Early AF ablation in a contemporary prospective cohort of AF patients who were predominantly treated with NOACs was associated with a survival advantage compared to medical therapy alone.
Trial registration
Clinical trial registration: http://www.clinicaltrials.gov. Unique identifiers: NCT01468701, NCT01671007 and NCT01937377.
Graphical abstract

Created with BioRender.com.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is a cardiac arrhythmia that is characterised by an irregular heart beat and has important interactions with numerous other conditions. It is associated with an increased risk of thromboembolic complications, heart failure and death [1,2,3], and excess healthcare costs [4]. The treatment of patients with AF includes the use of either rhythm or rate control management strategies to achieve symptom control, as per current international guidelines [5,6,7]. These strategies were accepted as equivocal on the basis of historical studies which demonstrated similar outcomes with both [8]. Nonetheless, this notion has been challenged by more recent evidence from the EAST–AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention) trial suggesting that early rhythm control may offer a prognostic advantage over rate control [9].
Rhythm control may be achieved using either cardioversion with anti-arrhythmic drugs or electrical therapy, or AF ablation. Over the past decade, the superiority of AF ablation over anti-arrhythmic drugs for the prevention of atrial arrhythmia recurrence has been proven [10], such that there is now an increasing argument for its use as first-line treatment in patients with AF. Recently, we published a systematic review and meta-analysis of 6 randomised controlled trials demonstrating that first-line treatment with catheter AF ablation was associated with a 36% reduction in the recurrence of atrial arrhythmias and 47% reduction in healthcare resource utilisation compared to anti-arrhythmic drug therapy [11].
Despite the advantages of AF ablation as described above, its benefits among the general AF population in terms of hard clinical outcomes including stroke, heart failure and long-term survival remain ill-defined. Several observational studies previously reported a prognostic benefit with AF ablation [12,13,14,15], though this was not found in the CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation) randomised controlled trial [10]. Nonetheless, the aforementioned studies were not designed to test for the effects of early AF ablation and only 8% of patients in the early rhythm control arm of EAST–AFNET 4 received such treatment [9]. Herein, we aimed to evaluate the effects of early AF ablation in patients from the contemporary prospective GLORIA-AF (Global Registry on Long-Term Oral Anti-thrombotic Treatment In Patients With Atrial Fibrillation) registry.
Methods
Study design and population
GLORIA-AF is a prospective, observational, global registry programme of patients from 935 centres across 38 participating countries in Asia, Europe, North America, Latin America, and Africa/Middle East. The study design has previously been described [16]. In brief, consecutive adults with newly diagnosed AF (< 3 months before baseline visit) and an increased risk of stroke (CHA2DS2–VASc ≥ 1) were enrolled. This study focused on patients from GLORIA-AF phase II and III. These patients were enrolled between 2011 and 2020. Patients with known ablation status at baseline and follow-up data were included. Main exclusion criteria of GLORIA-AF registry were the presence of mechanical heart valve or valvular disease necessitating valve replacement, prior oral anticoagulation with vitamin K oral antagonist over 60 days, a reversible cause of AF, indication for anticoagulation other than AF, and life expectancy of less than 1 year. Ethics approval was obtained from the local institutional review boards, informed consent was obtained from patients, and the study was performed in accordance with the Declaration of Helsinki.
Data collection and definition
Data on demographics, comorbidities and therapies were collected at baseline with standardised, prospectively designed data collection tools. Early AF ablation was defined as AF ablation within 3 months from diagnosis. Creatinine clearance (CrCl) was assessed using the Cockcroft–Gault equation [17]. AF classification was determined according to the European Society of Cardiology recommendations [18]. Severity of AF-related symptoms was ascertained using the European Heart Rhythm Association classification [19]. CHADS2, CHA2DS2–VASc and HAS–BLED scores were calculated as previously described [20,21,22].
Study outcomes and follow-up
Outcomes of interest were the composite outcome of all-cause death, stroke and major bleeding, and the pre-specified outcomes of all-cause death, cardiovascular (CV) death, non-CV death, stroke and major bleeding. Stroke was defined as an acute onset of a focal neurological deficit of presumed vascular origin lasting for 24 h or more, or resulting in death. Major bleeding was defined as either overt bleeding associated with a reduction in haemoglobin of at least 20 g/L or leading to a transfusion of at least 2 units of blood or packed cells; symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding or pericardial bleeding); or life-threatening bleeding. In phase II, follow-up for the dabigatran cohort was for 2 years, with scheduled visits at 3, 6, 12, and 24 months. In phase III, follow-up for all patients was conducted for 3 years, with scheduled visits at 6, 12, 24, and 36 months.
Statistical analysis
Continuous variables were described with median and interquartile range (IQR), and tested for differences with Kruskal–Wallis test. Categorical variables were described as count and percentage, and tested for differences with chi-squared test. Plots of Kaplan–Meier curves were performed for each outcome and survival distributions were compared using log-rank test. Cox proportional hazards analyses were performed to study the effects of early AF ablation on outcomes of interest. Potential confounders were accounted for using a multivariable model with forward selection of covariates including age, gender, body mass index, CrCl, type of AF, hypertension, hyperlipidaemia, diabetes mellitus, coronary artery disease, heart failure, left ventricular hypertrophy, prior thromboembolism, prior bleeding, peripheral artery disease, chronic obstructive pulmonary disease, oral anticoagulation use, antiplatelet use, anti-arrhythmic drug therapy, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, digoxin, statin and diuretic therapy.
Further analyses were undertaken after rigorous adjustment for baseline characteristics with propensity score matching (PSM) generated by logistic regression for all variables in Supplementary Table 1 in a 1:1 ratio using the nearest-neighbour technique without replacement. A two-sided p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using RStudio (Version 1.3.1093).
Results
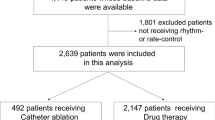
A total of 25,963 patients (11,733 [45.2%] females; median age 71 [IQR 64–78] years) were included. A flow chart of the patient selection process is shown in Supplementary Fig. 1. The anticoagulation status of patients in this study cohort is demonstrated in Fig. 1. There were 445 (1.7%) patients treated with early AF ablation and 25,518 (98.3%) with medical therapy.
Baseline characteristics
Baseline characteristics are described in Table 1. Patients in the early AF ablation group were younger and had lower body mass index, higher CrCl, less advanced forms of AF and reduced burden of comorbidities including hypertension, hypercholesterolaemia, diabetes mellitus, prior myocardial infarction, heart failure, left ventricular hypertrophy, prior thromboembolism and chronic obstructive pulmonary disease compared to patients on medical therapy alone. As a result, patients treated with early AF ablation had a lower risk of stroke (median CHADS2 of 1 [IQR 1–2] vs. 2 [IQR 1–3]; CHA2DS2–VASc of 2 [IQR 1–3] vs. 3 [IQR 2–4]) and major bleeding (median HAS–BLED of 1 [IQR 0–1] vs. 1 [IQR 1–2]).
Medication use
Oral anticoagulation was prescribed in 22,219 (85.6%) patients at baseline with 17,424 (67.1%) receiving non-vitamin K antagonist oral anticoagulants and 4795 (18.5%) vitamin K antagonist. In contrast to patients on medical therapy, those treated with early AF ablation had greater uptake of anticoagulation and anti-arrhythmic drug therapy but less frequent use of antiplatelet agent, angiotensin-converting enzyme inhibitor, beta-blocker, digoxin and diuretic therapy (Table 2).
Study outcomes
During a median follow-up period of 3.0 (IQR 2.3–3.1) years, there were 3237 (12.5%) events for the composite outcome of all-cause death, stroke and major bleeding, including 2305 (8.9%) all-cause deaths, 807 (3.1%) CV deaths, 989 (3.8%) non-CV deaths, 679 (2.6%) strokes and 870 (3.4%) major bleeding events. Kaplan–Meier survival analyses demonstrated that patients who were treated with early AF ablation had lower event rates for the composite outcome, all-cause death, CV death, non-CV death and major bleeding with a trend for reduced stroke compared to patients on medical therapy alone (Fig. 2).
Early AF ablation was associated with a significant reduction in the composite outcome of all-cause death, stroke and major bleeding (HR 0.26 [95% CI, 0.16–0.43]), all-cause death (HR 0.22 [95% CI, 0.11–0.42]), CV death (HR 0.21 [95% CI, 0.07–0.65]), non-CV death (HR 0.28 [95% CI, 0.11–0.66]), stroke (HR 0.43 [95% CI, 0.18–1.00]) and major bleeding (HR 0.39 [95% CI, 0.18–0.88]) (Table 3). After adjustment for various confounders, the composite outcome (HR 0.50 [95% CI, 0.30–0.85]) and all-cause death (HR 0.45 [95% CI, 0.23–0.91]) remained significantly lower among patients who were treated with early AF ablation compared to those on medical therapy.
A comparison of early AF ablation to other risk factors for the composite outcome of all-cause death, stroke and major bleeding are shown in Fig. 3. Worse outcomes were seen in patients with increased age, reduced renal function, diabetes mellitus, coronary artery disease, heart failure, prior thromboembolism, prior bleeding, peripheral artery disease, chronic obstructive pulmonary disease, antiplatelet use, digoxin and diuretic therapy. Protective factors were early AF ablation, female sex, oral anticoagulation use, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker and anti-arrhythmic drug therapy.
Effects of early AF ablation in comparison to other risk factors for the composite outcome of all-cause death, stroke and major bleeding. AAD anti-arrhythmic drug, ACE-i angiotensin-converting enzyme inhibitor, AF atrial fibrillation, ARB angiotensin receptor blocker, BMI body mass index, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, CrCl creatinine clearance, DM diabetes mellitus, HF heart failure, HTN hypertension, LVH left ventricular hypertrophy, OAC oral anticoagulation, PAD peripheral artery disease, TE thromboembolism
PSM cohort
Using PSM, we identified 399 patients in each group with comparable baseline characteristics (Supplementary Table 1). Within this cohort, early AF ablation was related to a decrease in the composite outcome of all-cause death, stroke and major bleeding (HR 0.41 [95% CI, 0.22–0.77]) and all-cause death (HR 0.43 [95% CI, 0.19–0.99]) (Table 4). There was no statistically significant difference between the groups in terms of CV death, non-CV death, stroke and major bleeding.
Discussion
In this large, global, prospective registry of patients with newly diagnosed AF who were predominantly treated with non-vitamin K antagonist oral anticoagulants, we present novel findings demonstrating that early AF ablation was independently associated with a 51% decrease in the composite outcome of all-cause death, stroke and major bleeding, and a 59% decrease in all-cause death compared to medical therapy alone over a 3-year follow-up period. Second, early AF ablation had the greatest benefit in terms of reducing the composite outcome vs. other therapies, such as oral anticoagulation; however, less than 2% of all patients with newly diagnosed AF received such treatment in this cohort.
The benefits of ablation therapy among patients with AF have previously been reported in observational studies across different centres and countries. A retrospective study using MarketScan administrative claims data showed that patients who were treated with AF ablation had a reduced risk of thromboembolic events compared to those on anti-arrhythmic drugs [12]. Another large population-based study from the United States found that patients with AF who were treated with an ablation procedure had significantly lower all-cause death, ischaemic stroke and haemorrhagic stroke compared to matched controls [13]. A propensity score-matched population-based study from Israel of patients with AF and predominantly a high CHA2DS2–VASc score found that catheter ablation was associated with a decreased risk of stroke or transient ischaemic attack, and all-cause death [14]. Another recent study using the Korean National Health Insurance database demonstrated that catheter AF ablation compared to medical therapy significantly reduced the risk of both ischaemic stroke and intracranial haemorrhage [23]. Furthermore, patients treated with catheter AF ablation had similar outcomes as the non-AF population. However, all of these retrospective studies suffer from potential selection bias which may have influenced the results in favour of AF ablation [24].
In contrast, the CABANA randomised trial found that there was no significant difference in the primary composite outcome of death, disabling stroke, serious bleeding or cardiac arrest between catheter ablation and medical therapy in patients with AF [10]. This trial was, however, plagued by several issues including cross-over of a considerable number of patients from medical therapy to catheter AF ablation, significant loss to follow-up or withdrawal, and lower than expected event rates which may have substantially compromised the validity of the results. Interestingly, pre-specified as-treated analyses demonstrated that the primary composite outcome was reduced with catheter AF ablation, as was the risk of death [10].
Given the differences in findings between observational studies and randomised controlled trials, it is perhaps unsurprising that there were conflicting results between meta-analyses on this topic, depending on the study selection criteria. In a meta-analysis of 11 randomised controlled trials comprised of 1763 patients, catheter AF ablation compared to anti-arrhythmic drug therapy led to a reduction in AF recurrence and improvement in quality of life but failed to alter the risk of all-cause death and stroke or transient ischaemic attack [25]. Another meta-analysis of 13 randomised controlled trials comprised of 3856 patients without heart failure also reported no significant difference between catheter AF ablation and medical therapy in terms of all-cause death and stroke [26]. However, patients who received catheter AF ablation had reduced cardiac hospitalisation and less frequent atrial arrhythmia recurrence, at the expense of procedural complications, such as pericardial tamponade; highlighting the need to recognise the invasive nature of AF ablation and to balance any intended benefits with the risk of potential complications [27]. Conversely, a meta-analysis of 9 studies (8 matched population studies and 1 randomised controlled trial) with 241,372 patients with AF found that catheter ablation decreased the risk of death, stroke and hospitalisation for heart failure compared to medical therapy alone [28].
Unlike the aforementioned studies, we investigated the effects of early AF ablation (i.e., within 3 months from diagnosis) in a prospectively enrolled global AF population with a high uptake of oral anticoagulation and found a significant survival benefit in these patients compared to those who received medical therapy alone. While we found a reduction in the risk of all-cause death with early AF ablation, we were unable to attribute this to CV or non-CV death likely owing to the low event rates. Notably, only a minority of patients were treated with early AF ablation and there were significant differences between the groups at baseline. Nonetheless, our results provide further support for the emerging role of early AF ablation and complements existing evidence from the EAST-AFNET 4 trial [9].
Current recommendations from international guidelines advocate that AF ablation should be reserved for patients who have failed at least 1 anti-arrhythmic drug therapy, though it may be considered in selected patients with early forms of AF or heart failure with reduced ejection fraction [5,6,7]. Healthcare structures in most countries are such that there is often a delay between the diagnosis of AF and specialist review, and/or subsequent referral for consideration of AF ablation. As a result, adoption of early AF ablation is uncommon, as observed in the present study. However, there is growing evidence to suggest that AF ablation should be considered early in the patient journey, consistent with the understanding that atrial remodelling occurs with chronic disease and hence the phrase ‘AF begets AF’ [29]. Furthermore, the importance of maintaining sinus rhythm should not be underestimated [30] and the success of catheter AF ablation is reduced with treatment delay [31]. To this end, systematic improvements are needed to facilitate the delivery of early AF ablation to patients who are eligible and may benefit from such treatment. Such facilitation is an argument for a more integrated care approach to patient care pathways, including for those AF and other chronic cardiovascular conditions [32, 33]. Indeed, adherence to a holistic or integrated care approach to AF care is associated with improved clinical outcomes [34, 35].
Limitations
The main limitations of this study are linked to possible misclassification and selection bias due its observational nature; though consecutive enrolment of patients was performed to reduce selection bias. As only a small proportion of patients were treated with early AF ablation, the results of this highly selected group may not be generalisable to the wider AF population. Despite rigorous model adjustment and propensity matching to ensure a balance of comorbidities and medication use between the groups, some residual unmeasured confounders may exist. Therefore, we are unable to prove a cause–effect relationship. In addition, outcomes were analysed according to variables collected at baseline. In this regard, a proportion of patients in the medical therapy group may have been treated with AF ablation following enrolment. This may have attenuated any differences between the groups but serves to provide further strength to our positive findings. Overall, the results of this post-hoc analysis should be treated as hypotheses-generating.
Conclusions
Early AF ablation within 3 months from initial diagnosis in a contemporary cohort of patients who were predominantly treated with non-vitamin K antagonist oral anticoagulants was associated with a survival advantage compared to medical therapy alone. Moreover, early AF ablation appeared to provide the greatest benefit compared to other treatments.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Benjamin EJ, Wolf PA, D’Agostino RB et al (1998) Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation 98:946–952. https://doi.org/10.1161/01.CIR.98.10.946
Stewart S, Hart CL, Hole DJ, McMurray JJ (2002) A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 113:359–364. https://doi.org/10.1016/s0002-9343(02)01236-6
Vermond RA, Geelhoed B, Verweij N et al (2015) Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality a community-based study from the Netherlands. J Am Coll Cardiol 66:1000–1007. https://doi.org/10.1016/j.jacc.2015.06.1314
Burdett P, Lip GYH (2020) Atrial fibrillation in the United Kingdom: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation-related costs. Eur Hear J-Qual Care Clin Outcomes. https://doi.org/10.1093/ehjqcco/qcaa093
Hindricks G, Potpara T, Dagres N et al (2021) 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio-thoracic surgery (EACTS). Eur Heart J 42:373–498. https://doi.org/10.1093/eurheartj/ehaa612
January CT, Wann LS, Alpert JS et al (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation 130:2071–2104. https://doi.org/10.1161/CIR.0000000000000040
January CT, Wann LS, Calkins H et al (2019) 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart R. J Am Coll Cardiol 74:104–132. https://doi.org/10.1016/j.jacc.2019.01.011
Sethi NJ, Feinberg J, Nielsen EE et al (2017) The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: a systematic review with meta-analysis and trial sequential analysis. PLoS One 12:e0186856. https://doi.org/10.1371/journal.pone.0186856
Kirchhof P, Camm AJ, Goette A et al (2020) Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 383:1305–1316. https://doi.org/10.1056/NEJMoa2019422
Packer DL, Mark DB, Robb RA et al (2019) Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 321:1261–1274. https://doi.org/10.1001/jama.2019.0693
Imberti JF, Ding WY, Kotalczyk A et al (2021) Catheter ablation as first-line treatment for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Heart 107:1630–1636. https://doi.org/10.1136/heartjnl-2021-319496
Mansour M, Heist EK, Agarwal R et al (2018) Stroke and cardiovascular events after ablation or antiarrhythmic drugs for treatment of patients with atrial fibrillation. Am J Cardiol 121:1192–1199. https://doi.org/10.1016/j.amjcard.2018.01.043
Srivatsa UN, Danielsen B, Amsterdam EA et al (2018) CAABL-AF (California study of ablation for atrial fibrillation): mortality and stroke, 2005 to 2013. Circ Arrhythm Electrophysiol 11:e005739. https://doi.org/10.1161/CIRCEP.117.005739
Saliba W, Schliamser JE, Lavi I et al (2017) Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: a propensity score-matched analysis. Hear Rhythm 14:635–642. https://doi.org/10.1016/j.hrthm.2017.02.001
Ding WY, Williams E, Das M et al (2021) Cryoballoon pulmonary vein isolation as first line treatment for typical atrial flutter (CRAFT): study protocol for a randomised controlled trial. J Interv Card Electrophysiol 60:427–432. https://doi.org/10.1007/s10840-020-00746-6
Huisman MV, Lip GYH, Diener HC et al (2014) Design and rationale of global registry on long-term oral antithrombotic treatment in patients with atrial fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J 167:329–334. https://doi.org/10.1016/j.ahj.2013.12.006
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. https://doi.org/10.1159/000180580
Kirchhof P, Benussi S, Kotecha D et al (2016) 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37:2893–2962. https://doi.org/10.1093/eurheartj/ehw210
Kirchhof P, Auricchio A, Bax J et al (2007) Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German atrial fibrillation competence NETwork and the European heart rhythm association. Europace 9:1006–1023. https://doi.org/10.1093/europace/eum191
Lip GYH, Nieuwlaat R, Pisters R et al (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 137:263–272. https://doi.org/10.1378/chest.09-1584
Pisters R, Lane DA, Nieuwlaat R et al (2010) A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 138:1093–1100. https://doi.org/10.1378/chest.10-0134
Gage BF, Waterman AD, Shannon W et al (2001) Validation of clinical classification schemes for predicting stroke: results from the National registry of atrial fibrillation. JAMA 285:2864–2870. https://doi.org/10.1001/jama.285.22.2864
Kim M, Yu HT, Kim J et al (2021) Atrial fibrillation and the risk of ischaemic strokes or intracranial haemorrhages: comparisons of the catheter ablation, medical therapy, and non-atrial fibrillation population. Europace 23:529–538. https://doi.org/10.1093/europace/euaa235
Ding WY, Gupta D (2021) Catheter ablation: the “Pym particles” of atrial fibrillation? Europace 23:489–490. https://doi.org/10.1093/europace/euaa299
Shi L-Z, Heng R, Liu S-M, Leng F-Y (2015) Effect of catheter ablation versus antiarrhythmic drugs on atrial fibrillation: a meta-analysis of randomized controlled trials. Exp Ther Med 10:816–822. https://doi.org/10.3892/etm.2015.2545
Muhammad ZK, Safi UK, Adeel A et al (2020) Meta-analysis of catheter ablation versus medical therapy in patients with atrial fibrillation without heart failure. J Atr Fibrillation 12:2266. https://doi.org/10.4022/jafib.2266
Gupta A, Perera T, Ganesan A et al (2013) Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol 6:1082–1088. https://doi.org/10.1161/CIRCEP.113.000768
Saglietto A, De Ponti R, Di Biase L et al (2020) Impact of atrial fibrillation catheter ablation on mortality, stroke, and heart failure hospitalizations: a meta-analysis. J Cardiovasc Electrophysiol 31:1040–1047. https://doi.org/10.1111/jce.14429
Nattel S, Harada M (2014) Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 63:2335–2345. https://doi.org/10.1016/j.jacc.2014.02.555
Verma A, Natale A (2005) Should atrial fibrillation ablation be considered first-line therapy for some patients? Why atrial fibrillation ablation should be considered first-line therapy for some patients. Circulation 112:1214–22. https://doi.org/10.1161/CIRCULATIONAHA.104.478263
Chew DS, Black-Maier E, Loring Z et al (2020) Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol 13:e008128. https://doi.org/10.1161/CIRCEP.119.008128
Lip GYH, Ntaios G (2021) “Novel clinical concepts in thrombosis”: integrated care for stroke management-easy as ABC. Thromb Haemost. https://doi.org/10.1055/a-1632-1777
Field M, Kuduvalli M, Torella F et al (2021) Integrated care systems and the aortovascular hub. Thromb Haemost. https://doi.org/10.1055/a-1591-8033
Yoon M, Yang P-S, Jang E et al (2019) Improved population-based clinical outcomes of patients with atrial fibrillation by compliance with the simple abc (atrial fibrillation better care) pathway for integrated care management: a nationwide cohort study. Thromb Haemost 119:1695–1703. https://doi.org/10.1055/s-0039-1693516
Romiti GF, Pastori D, Rivera-Caravaca JM et al (2021) Adherence to the “atrial fibrillation better care” pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost. https://doi.org/10.1055/a-1515-9630
Acknowledgements
This publication is based on research using data from Boehringer Ingelheim that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. The authors thank the patients who participated in this trial, their families, the investigators, study co-ordinators, and study teams. GLORIA-AF Investigators are listed below: Dzifa Wosornu Abban, Nasser Abdul, Atilio Marcelo Abud, Fran Adams, Srinivas Addala, Pedro Adragão, Walter Ageno, Rajesh Aggarwal, Sergio Agosti, Piergiuseppe Agostoni, Francisco Aguilar, Julio Aguilar Linares, Luis Aguinaga, Jameel Ahmed, Allessandro Aiello, Paul Ainsworth, Jorge Roberto Aiub, Raed Al-Dallow, Lisa Alderson, Jorge Antonio Aldrete Velasco, Dimitrios Alexopoulos, Fernando Alfonso Manterola, Pareed Aliyar, David Alonso, Fernando Augusto Alves da Costa, José Amado, Walid Amara, Mathieu Amelot, Nima Amjadi, Fabrizio Ammirati, Marianna Andrade, Nabil Andrawis, Giorgio Annoni, Gerardo Ansalone, M.Kevin Ariani, Juan Carlos Arias, Sébastien Armero, Chander Arora, Muhammad Shakil Aslam, M. Asselman, Philippe Audouin, Charles Augenbraun, S. Aydin, Ivaneta Ayryanova, Emad Aziz, Luciano Marcelo Backes, E. Badings, Ermentina Bagni, Seth H. Baker, Richard Bala, Antonio Baldi, Shigenobu Bando, Subhash Banerjee, Alan Bank, Gonzalo Barón Esquivias, Craig Barr, Maria Bartlett, Vanja Basic Kes, Giovanni Baula, Steffen Behrens, Alan Bell, Raffaella Benedetti, Juan Benezet Mazuecos, Bouziane Benhalima, Jutta Bergler-Klein, Jean-Baptiste Berneau, Richard A. Bernstein, Percy Berrospi, Sergio Berti, Andrea Berz, Elizabeth Best, Paulo Bettencourt, Robert Betzu, Ravi Bhagwat, Luna Bhatta, Francesco Biscione, Giovanni Bisignani, Toby Black, Michael J. Bloch, Stephen Bloom, Edwin Blumberg, Mario Bo, Ellen Bøhmer, Andreas Bollmann, Maria Grazia Bongiorni, Giuseppe Boriani, D.J. Boswijk, Jochen Bott, Edo Bottacchi, Marica Bracic Kalan, Drew Bradman, Donald Brautigam, Nicolas Breton, P.J.A.M. Brouwers, Kevin Browne, Jordi Bruguera Cortada, A. Bruni, Claude Brunschwig, Hervé Buathier, Aurélie Buhl, John Bullinga, Jose Walter Cabrera, Alberto Caccavo, Shanglang Cai, Sarah Caine, Leonardo Calò, Valeria Calvi, Mauricio Camarillo Sánchez, Rui Candeias, Vincenzo Capuano, Alessandro Capucci, Ronald Caputo, Tatiana Cárdenas Rizo, Francisco Cardona, Francisco Carlos da Costa Darrieux, Yan Carlos Duarte Vera, Antonio Carolei, Susana Carreño, Paula Carvalho, Susanna Cary, Gavino Casu, Claudio Cavallini, Guillaume Cayla, Aldo Celentano, Tae-Joon Cha, Kwang Soo Cha, Jei Keon Chae, Kathrine Chalamidas, Krishnan Challappa, Sunil Prakash Chand, Harinath Chandrashekar, Ludovic Chartier, Kausik Chatterjee, Carlos Antero Chavez Ayala, Aamir Cheema, Amjad Cheema, Lin Chen, Shih-Ann Chen, Jyh Hong Chen, Fu-Tien Chiang, Francesco Chiarella, Lin Chih-Chan, Yong Keun Cho, Jong-Il Choi, Dong Ju Choi, Guy Chouinard, Danny Hoi-Fan Chow, Dimitrios Chrysos, Galina Chumakova, Eduardo Julián José Roberto Chuquiure Valenzuela, Nicoleta Cindea Nica, David J. Cislowski, Anthony Clay, Piers Clifford, Andrew Cohen, Michael Cohen, Serge Cohen, Furio Colivicchi, Ronan Collins, Paolo Colonna, Steve Compton, Derek Connolly, Alberto Conti, Gabriel Contreras Buenostro, Gregg Coodley, Martin Cooper, Julian Coronel, Giovanni Corso, Juan Cosín Sales, Yves Cottin, John Covalesky, Aurel Cracan, Filippo Crea, Peter Crean, James Crenshaw, Tina Cullen, Harald Darius, Patrick Dary, Olivier Dascotte, Ira Dauber, Vicente Davalos, Ruth Davies, Gershan Davis, Jean-Marc Davy, Mark Dayer, Marzia De Biasio, Silvana De Bonis, Raffaele De Caterina, Teresiano De Franceschi, J.R. de Groot, José De Horta, Axel De La Briolle, Gilberto de la Pena Topete, Angelo Amato Vicenzo de Paola, Weimar de Souza, A. de Veer, Luc De Wolf, Eric Decoulx, Sasalu Deepak, Pascal Defaye, Freddy Del-Carpio Munoz, Diana Delic Brkljacic, N. Joseph Deumite, Silvia Di Legge, Igor Diemberger, Denise Dietz, Pedro Dionísio, Qiang Dong, Fabio Rossi dos Santos, Elena Dotcheva, Rami Doukky, Anthony D'Souza, Simon Dubrey, Xavier Ducrocq, Dmitry Dupljakov, Mauricio Duque, Dipankar Dutta, Nathalie Duvilla, A. Duygun, Rainer Dziewas, Charles B. Eaton, William Eaves, L.A Ebels-Tuinbeek, Clifford Ehrlich, Sabine Eichinger-Hasenauer, Steven J. Eisenberg, Adnan El Jabali, Mahfouz El Shahawy, Mauro Esteves Hernandes, Ana Etxeberria Izal, Rudolph Evonich III, Oksana Evseeva, Andrey Ezhov, Raed Fahmy, Quan Fang, Ramin Farsad, Laurent Fauchier, Stefano Favale, Maxime Fayard, Jose Luis Fedele, Francesco Fedele, Olga Fedorishina, Steven R. Fera, Luis Gustavo Gomes Ferreira, Jorge Ferreira, Claudio Ferri, Anna Ferrier, Hugo Ferro, Alexandra Finsen, Brian First, Stuart Fischer, Catarina Fonseca, Luísa Fonseca Almeida, Steven Forman, Brad Frandsen, William French, Keith Friedman, Athena Friese, Ana Gabriela Fruntelata, Shigeru Fujii, Stefano Fumagalli, Marta Fundamenski, Yutaka Furukawa, Matthias Gabelmann, Nashwa Gabra, Niels Gadsbøll, Michel Galinier, Anders Gammelgaard, Priya Ganeshkumar, Christopher Gans, Antonio Garcia Quintana, Olivier Gartenlaub, Achille Gaspardone, Conrad Genz, Frédéric Georger, Jean-Louis Georges, Steven Georgeson, Evaldas Giedrimas, Mariusz Gierba, Ignacio Gil Ortega, Eve Gillespie, Alberto Giniger, Michael C. Giudici, Alexandros Gkotsis, Taya V. Glotzer, Joachim Gmehling, Jacek Gniot, Peter Goethals, Seth Goldbarg, Ronald Goldberg, Britta Goldmann, Sergey Golitsyn, Silvia Gómez, Juan Gomez Mesa, Vicente Bertomeu Gonzalez, Jesus Antonio Gonzalez Hermosillo, Víctor Manuel González López, Hervé Gorka, Charles Gornick, Diana Gorog, Venkat Gottipaty, Pascal Goube, Ioannis Goudevenos, Brett Graham, G. Stephen Greer, Uwe Gremmler, Paul G. Grena, Martin Grond, Edoardo Gronda, Gerian Grönefeld, Xiang Gu, Ivett Guadalupe Torres Torres, Gabriele Guardigli, Carolina Guevara, Alexandre Guignier, Michele Gulizia, Michael Gumbley, Albrecht Günther, Andrew Ha, Georgios Hahalis, Joseph Hakas, Christian Hall, Bing Han, Seongwook Han, Joe Hargrove, David Hargroves, Kenneth B. Harris, Tetsuya Haruna, Emil Hayek, Jeff Healey, Steven Hearne, Michael Heffernan, Geir Heggelund, J.A. Heijmeriks, Maarten Hemels, I. Hendriks, Sam Henein, Sung-Ho Her, Paul Hermany, Jorge Eduardo Hernández Del Río, Yorihiko Higashino, Michael Hill, Tetsuo Hisadome, Eiji Hishida, Etienne Hoffer, Matthew Hoghton, Kui Hong, Suk keun Hong, Stevie Horbach, Masataka Horiuchi, Yinglong Hou, Jeff Hsing, Chi-Hung Huang, David Huckins, kathy Hughes, A. Huizinga, E.L. Hulsman, Kuo-Chun Hung, Gyo-Seung Hwang, Margaret Ikpoh, Davide Imberti, Hüseyin Ince, Ciro Indolfi, Shujiro Inoue, Didier Irles, Harukazu Iseki, C. Noah Israel, Bruce Iteld, Venkat Iyer, Ewart Jackson-Voyzey, Naseem Jaffrani, Frank Jäger, Martin James, Sung-Won Jang, Nicolas Jaramillo, Nabil Jarmukli, Robert J. Jeanfreau, Ronald D. Jenkins, Carlos Jerjes Sánchez, Javier Jimenez, Robert Jobe, Tomas Joen-Jakobsen, Nicholas Jones, Jose Carlos Moura Jorge, Bernard Jouve, Byung Chun Jung, Kyung Tae Jung, Werner Jung, Mikhail Kachkovskiy, Krystallenia Kafkala, Larisa Kalinina, Bernd Kallmünzer, Farzan Kamali, Takehiro Kamo, Priit Kampus, Hisham Kashou, Andreas Kastrup, Apostolos Katsivas, Elizabeth Kaufman, Kazuya Kawai, Kenji Kawajiri, John F. Kazmierski, P Keeling, José Francisco Kerr Saraiva, Galina Ketova, Ajit Singh Khaira, Aleksey Khripun, Doo-Il Kim, Young Hoon Kim, Nam Ho Kim, Dae Kyeong Kim, Jeong Su Kim, June Soo Kim, Ki Seok Kim, Jin bae Kim, Elena Kinova, Alexander Klein, James J. Kmetzo, G. Larsen Kneller, Aleksandar Knezevic, Su Mei Angela Koh, Shunichi Koide, Athanasios Kollias, J.A. Kooistra, Jay Koons, Martin Koschutnik, William J. Kostis, Dragan Kovacic, Jacek Kowalczyk, Natalya Koziolova, Peter Kraft, Johannes A. Kragten, Mori Krantz, Lars Krause, B.J. Krenning, F. Krikke, Z. Kromhout, Waldemar Krysiak, Priya Kumar, Thomas Kümler, Malte Kuniss, Jen-Yuan Kuo, Achim Küppers, Karla Kurrelmeyer, Choong Hwan Kwak, Bénédicte Laboulle, Arthur Labovitz, Wen Ter Lai, Andy Lam, Yat Yin Lam, Fernando Lanas Zanetti, Charles Landau, Giancarlo Landini, Estêvão Lanna Figueiredo, Torben Larsen, Karine Lavandier, Jessica LeBlanc, Moon Hyoung Lee, Chang-Hoon Lee, John Lehman, Ana Leitão, Nicolas Lellouche, Malgorzata Lelonek, Radoslaw Lenarczyk, T. Lenderink, Salvador León González, Peter Leong-Sit, Matthias Leschke, Nicolas Ley, Zhanquan Li, Xiaodong Li, Weihua Li, Xiaoming Li, Christhoh Lichy, Ira Lieber, Ramon Horacio Limon Rodriguez, Hailong Lin, Feng Liu, Hengliang Liu, Guillermo Llamas Esperon, Nassip Llerena Navarro, Eric Lo, Sergiy Lokshyn, Amador López, José Luís López-Sendón, Adalberto Menezes Lorga Filho, Richard S. Lorraine, Carlos Alberto Luengas, Robert Luke, Ming Luo, Steven Lupovitch, Philippe Lyrer, Changsheng Ma, Genshan Ma, Irene Madariaga, Koji Maeno, Dominique Magnin, Gustavo Maid, Sumeet K. Mainigi, Konstantinos Makaritsis, Rohit Malhotra, Rickey Manning, Athanasios Manolis, Helard Andres Manrique Hurtado, Ioannis Mantas, Fernando Manzur Jattin, Vicky Maqueda, Niccolo Marchionni, Francisco Marin Ortuno, Antonio Martín Santana, Jorge Martinez, Petra Maskova, Norberto Matadamas Hernandez, Katsuhiro Matsuda, Tillmann Maurer, Ciro Mauro, Erik May, Nolan Mayer, John McClure, Terry McCormack, William McGarity, Hugh McIntyre, Brent McLaurin, Feliz Alvaro Medina Palomino, Francesco Melandri, Hiroshi Meno, Dhananjai Menzies, Marco Mercader, Christian Meyer, Beat j. Meyer, Jacek Miarka, Frank Mibach, Dominik Michalski, Patrik Michel, Rami Mihail Chreih, Ghiath Mikdadi, Milan Mikus, Davor Milicic, Constantin Militaru, Sedi Minaie, Bogdan Minescu, Iveta Mintale, Tristan Mirault, Michael J. Mirro, Dinesh Mistry, Nicoleta Violeta Miu, Naomasa Miyamoto, Tiziano Moccetti, Akber Mohammed, Azlisham Mohd Nor, Michael Mollerus, Giulio Molon, Sergio Mondillo, Patrícia Moniz, Lluis Mont, Vicente Montagud, Oscar Montaña, Cristina Monti, Luciano Moretti, Kiyoo Mori, Andrew Moriarty, Jacek Morka, Luigi Moschini, Nikitas Moschos, Andreas Mügge, Thomas J. Mulhearn, Carmen Muresan, Michela Muriago, Wlodzimierz Musial, Carl W. Musser, Francesco Musumeci, Thuraia Nageh, Hidemitsu Nakagawa, Yuichiro Nakamura, Toru Nakayama, Gi-Byoung Nam, Michele Nanna, Indira Natarajan, Hemal M. Nayak, Stefan Naydenov, Jurica Nazlić, Alexandru Cristian Nechita, Libor Nechvatal, Sandra Adela Negron, James Neiman, Fernando Carvalho Neuenschwander, David Neves, Anna Neykova, Ricardo Nicolás Miguel, George Nijmeh, Alexey Nizov, Rodrigo Noronha Campos, Janko Nossan, Tatiana Novikova, Ewa Nowalany-Kozielska, Emmanuel Nsah, Juan Carlos Nunez Fragoso, Svetlana Nurgalieva, Dieter Nuyens, Ole Nyvad, Manuel Odin de Los Rios Ibarra, Philip O'Donnell, Martin O'Donnell, Seil Oh, Yong Seog Oh, Dongjin Oh, Gilles O'Hara, Kostas Oikonomou, Claudia Olivares, Richard Oliver, Rafael Olvera Ruiz, Christoforos Olympios, Anna omaszuk-Kazberuk, Joaquín Osca Asensi, eena Padayattil jose, Francisco Gerardo Padilla Padilla, Victoria Padilla Rios, Giuseppe Pajes, A. Shekhar Pandey, Gaetano Paparella, F Paris, Hyung Wook Park, Jong Sung Park, Fragkiskos Parthenakis, Enrico Passamonti, Rajesh J. Patel, Jaydutt Patel, Mehool Patel, Janice Patrick, Ricardo Pavón Jimenez, Analía Paz, Vittorio Pengo, William Pentz, Beatriz Pérez, Alma Minerva Pérez Ríos, Alejandro Pérez-Cabezas, Richard Perlman, Viktor Persic, Francesco Perticone, Terri K. Peters, Sanjiv Petkar, Luis Felipe Pezo, Christian Pflücke, David N. Pham, Roland T. Phillips, Stephen Phlaum, Denis Pieters, Julien Pineau, Arnold Pinter, Fausto Pinto, R. Pisters, Nediljko Pivac, Darko Pocanic, Cristian Podoleanu, Alessandro Politano, Zdravka Poljakovic, Stewart Pollock, Jose Polo Garcéa, Holger Poppert, Maurizio Porcu, Antonio Pose Reino, Neeraj Prasad, Dalton Bertolim Précoma, Alessandro Prelle, John Prodafikas, Konstantin Protasov, Maurice Pye, Zhaohui Qiu, Jean-Michel Quedillac, Dimitar Raev, Carlos Antonio Raffo Grado, Sidiqullah Rahimi, Arturo Raisaro, Bhola Rama, Ricardo Ramos, Maria Ranieri, Nuno Raposo, Eric Rashba, Ursula Rauch-Kroehnert, Ramakota Reddy, Giulia Renda, Shabbir Reza, Luigi Ria, Dimitrios Richter, Hans Rickli, Werner Rieker, Tomas Ripolil Vera, Luiz Eduardo Ritt, Douglas Roberts, Ignacio Rodriguez Briones, Aldo Edwin Rodriguez Escudero, Carlos Rodríguez Pascual, Mark Roman, Francesco Romeo, E. Ronner, Jean-Francois Roux, Nadezda Rozkova, Miroslav Rubacek, Frank Rubalcava, Andrea M. Russo, Matthieu Pierre Rutgers, Karin Rybak, Samir Said, Tamotsu Sakamoto, Abraham Salacata, Adrien Salem, Rafael Salguero Bodes, Marco A. Saltzman, Alessandro Salvioni, Gregorio Sanchez Vallejo, Marcelo Sanmartín Fernández, Wladmir Faustino Saporito, Kesari Sarikonda, Taishi Sasaoka, Hamdi Sati, Irina Savelieva, Pierre-Jean Scala, Peter Schellinger, Carlos Scherr, Lisa Schmitz, Karl-Heinz Schmitz, Bettina Schmitz, Teresa Schnabel, Steffen Schnupp, Peter Schoeniger, Norbert Schön, Peter Schwimmbeck, Clare Seamark, Greg Searles, Karl-Heinz Seidl, Barry Seidman, Jaroslaw Sek, Lakshmanan Sekaran, Carlo Serrati, Neerav Shah, Vinay Shah, Anil Shah, Shujahat Shah, Vijay Kumar Sharma, Louise Shaw, Khalid H. Sheikh, Naruhito Shimizu, Hideki Shimomura, Dong-Gu Shin, Eun-Seok Shin, Junya Shite, Gerolamo Sibilio, Frank Silver, Iveta Sime, Tim A. Simmers, Narendra Singh, Peter Siostrzonek, Didier Smadja, David W. Smith, Marcelo Snitman, Dario Sobral Filho, Hassan Soda, Carl Sofley, Adam Sokal, Yannie Soo Oi Yan, Rodolfo Sotolongo, Olga Ferreira de Souza, Jon Arne Sparby, Jindrich Spinar, David Sprigings, Alex C. Spyropoulos, Dimitrios Stakos, Clemens Steinwender, Georgios Stergiou, Ian Stiell, Marcus Stoddard, Anastas Stoikov, Witold Streb, Ioannis Styliadis, Guohai Su, Xi Su, Wanda Sudnik, Kai Sukles, Xiaofei Sun, H. Swart, Janko Szavits-Nossan, Jens Taggeselle, Yuichiro Takagi, Amrit Pal Singh Takhar, Angelika Tamm, Katsumi Tanaka, Tanyanan Tanawuttiwat, Sherman Tang, Aylmer Tang, Giovanni Tarsi, Tiziana Tassinari, Ashis Tayal, Muzahir Tayebjee, J.M. ten Berg, Dan Tesloianu, Salem H.K. The, Dierk Thomas, Serge Timsit, Tetsuya Tobaru, Andrzej R. Tomasik., Mikhail Torosoff, Emmanuel Touze, Elina Trendafilova, W. Kevin Tsai, Hung Fat Tse, Hiroshi Tsutsui, Tian Ming Tu, Ype Tuininga, Minang Turakhia, Samir Turk, Wayne Turner, Arnljot Tveit, Richard Tytus, C Valadão, P.F.M.M. van Bergen, Philippe van de Borne, B.J. van den Berg, C van der Zwaan, M. Van Eck, Peter Vanacker, Dimo Vasilev, Vasileios Vasilikos, Maxim Vasilyev, Srikar Veerareddy, Mario Vega Miño, Asok Venkataraman, Paolo Verdecchia, Francesco Versaci, Ernst Günter Vester, Hubert Vial, Jason Victory, Alejandro Villamil, Marc Vincent, Anthony Vlastaris, Jürgen vom Dahl, Kishor Vora, Robert B. Vranian, Paul Wakefield, Ningfu Wang, Mingsheng Wang, Xinhua Wang, Feng Wang, Tian Wang, Alberta L. Warner, Kouki Watanabe, Jeanne Wei, Christian Weimar, Stanislav Weiner, Renate Weinrich, Ming-Shien Wen, Marcus Wiemer, Preben Wiggers, Andreas Wilke, David Williams, Marcus L. Williams, Bernhard Witzenbichler, Brian Wong, Ka Sing Lawrence Wong, Beata Wozakowska-Kaplon, Shulin Wu, Richard C. Wu, Silke Wunderlich, Nell Wyatt, John Jack Wylie, Yong Xu, Xiangdong Xu, Hiroki Yamanoue, Takeshi Yamashita, Ping Yen Bryan Yan, Tianlun Yang, Jing Yao, Kuo-Ho Yeh, Wei Hsian Yin, Yoto Yotov, Ralf Zahn, Stuart Zarich, Sergei Zenin, Elisabeth Louise Zeuthen, Huanyi Zhang, Donghui Zhang, Xingwei Zhang, Ping Zhang, Jun Zhang, Shui Ping Zhao, Yujie Zhao, Zhichen Zhao, Yang Zheng, Jing Zhou, Sergio Zimmermann, Andrea Zini, Steven Zizzo, Wenxia Zong, L Steven Zukerman.
Funding
The GLORIA-AF registry was sponsored by Boehringer Ingelheim GmbH. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Author information
Authors and Affiliations
Consortia
Contributions
DG: Speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Biosense Webster and Boston Scientific. Proctor for Abbott. Research Grants from Medtronic, Biosense Webster and Boston Scientific. MVH: Research grants from Dutch Healthcare Fund, Dutch Heart Foundation, Bayer Health Care, Pfizer-BMS, Leo Pharma, and consulting fees from Boehringer Ingelheim, Bayer Health Care, Pfizer-BMS. GYHL: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. Other authors declare no conflict of interest.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, W.Y., Calvert, P., Gupta, D. et al. Impact of early ablation of atrial fibrillation on long-term outcomes: results from phase II/III of the GLORIA-AF registry. Clin Res Cardiol 111, 1057–1068 (2022). https://doi.org/10.1007/s00392-022-02022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02022-1