Abstract
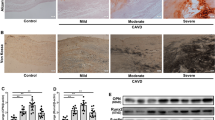
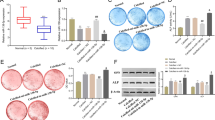
Calcific aortic valve disease (CAVD) is a complex active process involving in endothelial injury, lipid infiltration, chronic inflammation, matrix remodeling, cell differentiation, progressive bone formation, and new angiogenesis. The excess inflammatory responses induced by aortic valve interstitial cells (AVICs) are one of the common pathogeneses of this disease. Although many microRNAs (miRs) have been identified to play crucial roles in the calcification process of the aortic valve, numerous miRs are still waiting to be explored. In this study, we explored the functional role of miR-214 in the inflammatory reaction and calcification of human AVICs and its underlying molecular mechanism. Alizarin red staining was used to determine the number of calcified nodules. The protein levels of ICAM-1, IL-6, IL-8, and MCP-1 detected by enzyme-linked immunosorbent assay (ELISA) were used to assess the inflammatory reaction of AVICs; expression levels of RUNX2, Msx2, and BMP2 were used to evaluate AVICs osteoblast differentiation. Results showed that the expression levels of TLR4, MyD88, NF-κB, and miR-214 were up-regulated in the blood and aortic valve tissue samples of patients with CAVD when compared with normal individuals. Knockdown of miR-214 in AVICs inhibited the secretion of IL-6, IL-8, ICAM-1, and MCP-1, while this effect was repressed when lipopolysaccharide (LPS) was added to AVICs. LPS also enhanced the effects of miR-214 in promoting the secretion of pro-inflammatory factors. Besides, up-regulation of miR-214 promoted the protein expression of MyD88 and NF-κB but had no influence on TLR4, and miR-214 could directly combine with MyD88 protein. Up-regulation of MyD88 facilitated the secretion of pro-inflammatory factors and increased calcified nodules number and accelerated the expression of RUNX2, Msx2, and BMP2. Moreover, promotion of the expressions of pro-inflammatory factors and “osteoblast-like” cell markers induced by miR-214 overexpression was abolished when MyD88 was down-regulated in AVICs. In conclusion, this study revealed that miR-214 promoted calcification by facilitating inflammatory reaction through MyD88/NF-κB signaling pathway in AVICs.






Similar content being viewed by others
References
Coffey S, Cox B, Williams MJ (2014) The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 63(25 Pt A):2852–2861. https://doi.org/10.1016/j.jacc.2014.04.018
Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P (2016) Calcific aortic stenosis. Nat Rev Dis Primers 2:16006. https://doi.org/10.1038/nrdp.2016.6
Hutcheson JD, Aikawa E, Merryman WD (2014) Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol 11(4):218–231. https://doi.org/10.1038/nrcardio.2014.1
New SE, Aikawa E (2011) Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res 108(11):1381–1391. https://doi.org/10.1161/CIRCRESAHA.110.234146
Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD (1994) Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 90(2):844–853
Cote N, Mahmut A, Bosse Y, Couture C, Page S, Trahan S, Boulanger MC, Fournier D, Pibarot P, Mathieu P (2013) Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 36(3):573–581. https://doi.org/10.1007/s10753-012-9579-6
Weiss RM, Miller JD, Heistad DD (2013) Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res 113(2):209–222. https://doi.org/10.1161/CIRCRESAHA.113.300153
Mohler ER 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH (1999) Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 8(3):254–260
Lopez J, Fernandez-Pisonero I, Duenas AI, Maeso P, San Roman JA, Crespo MS, Garcia-Rodriguez C (2012) Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol 158(1):18–25. https://doi.org/10.1016/j.ijcard.2010.12.089
Zeng Q, Jin C, Ao L, Cleveland JC Jr, Song R, Xu D, Fullerton DA, Meng X (2012) Cross-talk between the Toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation 126(11 Suppl 1):S222–S230. https://doi.org/10.1161/CIRCULATIONAHA.111.083675
Yang X, Fullerton DA, Su X, Ao L, Cleveland JC Jr, Meng X (2009) Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of Toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J Am Coll Cardiol 53(6):491–500. https://doi.org/10.1016/j.jacc.2008.09.052
Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K (2011) Tumor necrosis factor-alpha accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther 337(1):16–23. https://doi.org/10.1124/jpet.110.177915
Alexopoulos A, Bravou V, Peroukides S, Kaklamanis L, Varakis J, Alexopoulos D, Papadaki H (2010) Bone regulatory factors NFATc1 and osterix in human calcific aortic valves. Int J Cardiol 139(2):142–149. https://doi.org/10.1016/j.ijcard.2008.10.014
Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA (2014) MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin 64(5):311–336. https://doi.org/10.3322/caac.21244
Pasquinelli AE (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13(4):271–282. https://doi.org/10.1038/nrg3162
Ambros V (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113(6):673–676
Fang M, Wang CG, Zheng C, Luo J, Hou S, Liu K, Li X (2017) Mir-29b promotes human aortic valve interstitial cell calcification via inhibiting TGF-beta3 through activation of wnt3/beta-catenin/Smad3 signaling. J Cell Biochem. https://doi.org/10.1002/jcb.26545
Li JP, Zhuang HT, Xin MY, Zhou YL (2017) MiR-214 inhibits human mesenchymal stem cells differentiating into osteoblasts through targeting beta-catenin. Eur Rev Med Pharmacol Sci 21(21):4777–4783
Messier RH Jr, Bass BL, Aly HM, Jones JL, Domkowski PW, Wallace RB, Hopkins RA (1994) Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J Surg Res 57(1):1–21. https://doi.org/10.1006/jsre.1994.1102
Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC Jr, Fullerton DA (2008) Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol 294(1):C29–C35. https://doi.org/10.1152/ajpcell.00137.2007
Mommer L, Wagemaker CA, Ouborg HDEK NJ (2008) Unravelling below-ground plant distributions: a real-time polymerase chain reaction method for quantifying species proportions in mixed root samples. Mol Ecol Resour 8(5):947–953. https://doi.org/10.1111/j.1755-0998.2008.02130.x
Ishizeki K, Saito H, Shinagawa T, Fujiwara N, Nawa T (1999) Histochemical and immunohistochemical analysis of the mechanism of calcification of Meckel’s cartilage during mandible development in rodents. J Anat 194(Pt 2):265–277
Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM (2011) Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease—2011 update. Circulation 124 (16):1783–1791. https://doi.org/10.1161/CIRCULATIONAHA.110.006767
Miller JD, Weiss RM, Heistad DD (2011) Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res 108(11):1392–1412. https://doi.org/10.1161/CIRCRESAHA.110.234138
Tsang HG, Cui L, Farquharson C, Corcoran BM, Summers KM, Macrae VE (2018) Exploiting novel valve interstitial cell lines to study calcific aortic valve disease. Mol Med Rep 17(2):2100–2106. https://doi.org/10.3892/mmr.2017.8163
Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B, Li H, Ma C (2013) MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting osterix. Bone 55(2):487–494. https://doi.org/10.1016/j.bone.2013.04.002
Li XF, Wang Y, Zheng DD, Xu HX, Wang T, Pan M, Shi JH, Zhu JH (2016) M1 macrophages promote aortic valve calcification mediated by microRNA-214/TWIST1 pathway in valvular interstitial cells. Am J Transl Res 8(12):5773–5783
Song Y, Fullerton DA, Mauchley D, Su X, Ao L, Yang X, Cleveland JC, Meng X (2011) Microfilaments facilitate TLR4-mediated ICAM-1 expression in human aortic valve interstitial cells. J Surg Res 166(1):52–58. https://doi.org/10.1016/j.jss.2009.03.101
Li GZ, Zhang Y, Zhao JB, Wu GJ, Su XF, Hang CH (2011) Expression of myeloid differentiation primary response protein 88 (Myd88) in the cerebral cortex after experimental traumatic brain injury in rats. Brain Res 1396:96–104. https://doi.org/10.1016/j.brainres.2011.04.014
Zeng Q, Song R, Ao L, Xu D, Venardos N, Fullerton DA, Meng X (2014) Augmented osteogenic responses in human aortic valve cells exposed to oxLDL and TLR4 agonist: a mechanistic role of Notch1 and NF-kappaB interaction. PLoS One 9(5):e95400. https://doi.org/10.1371/journal.pone.0095400
Deng X, Meng X, Zeng QC, Fullerton D, Jaggers J (2013) Abstract 15312: the inflammatory and osteogenic responses to Tlr4 stimulation in human aortic valve interstitial cells is greater in adults than children: the role of Stat3 as a negative regulatory mechanism. Circulation 128(22):A15312
El Husseini D, Boulanger MC, Mahmut A, Bouchareb R, Laflamme MH, Fournier D, Pibarot P, Bosse Y, Mathieu P (2014) P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol 72:146–156. https://doi.org/10.1016/j.yjmcc.2014.02.014
Song R, Ao L, Zhao KS, Zheng D, Venardos N, Fullerton DA, Meng X (2014) Soluble biglycan induces the production of ICAM-1 and MCP-1 in human aortic valve interstitial cells through TLR2/4 and the ERK1/2 pathway. Inflamm Res 63(9):703–710. https://doi.org/10.1007/s00011-014-0743-3
Kaisho T, Akira S (2006) Toll-like receptor function and signaling. J Allergy Clin Immunol 117(5):979–987. https://doi.org/10.1016/j.jaci.2006.02.023 (quiz 988)
Zhan Q, Zeng Q, Song R, Zhai Y, Xu D, Fullerton DA, Dinarello CA, Meng X (2017) IL-37 suppresses MyD88-mediated inflammatory responses in human aortic valve interstitial cells. Mol Med. https://doi.org/10.2119/molmed.2017.00022
Funding
This study was funded by the Science Project of Nantong (no. MS 22015077, no. MS 32015027) and Nantong University Teaching Reform Project (no. 2016B93).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zheng, D., Zang, Y., Xu, H. et al. MicroRNA-214 promotes the calcification of human aortic valve interstitial cells through the acceleration of inflammatory reactions with activated MyD88/NF-κB signaling. Clin Res Cardiol 108, 691–702 (2019). https://doi.org/10.1007/s00392-018-1398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1398-9