Abstract
Purpose
The aim of this study was to analyse the effect of an algorithm-based analgesic-sedative management on mechanical ventilation time and length of stay in a cardiological ICU with critical ill patients after sudden cardiac arrest.
Methods
We examined 100 patients after successful resuscitation in a retrospective-prospective single-centre trial by introducing an algorithm-based sedation management. Demographic data, severity of illness classified by APACHE II score (Acute Physiology and Chronic Health Evaluation II), neurological outcome and data for mechanical ventilation time and length of stay were acquired for both groups.
Results
We found a shorter ventilation time for young patients without severe illness, whereby significant longer ventilation time was observed for patients with higher APACHE II score. Between both groups, we found no significant differences in mechanical ventilation time and length of stay.
Conclusions
Our results demonstrate a tendency towards a reduction of mechanical ventilation time for patients without severe illness after sudden cardiac arrest achieved by implementation of a new sedation management, whereby significant longer ventilation time was observed for severe ill patients. Because of lack of statistical significance of our present study, a randomized study with sufficient power is necessary to demonstrate positive effects of a standardized sedation management and its influence on severity of illness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sedatives and analgesics play a key role in the therapy of critically ill patients, by reduction of anxiety, agitation and pain [1]. Duration and depth of sedation affects the mechanical ventilation time (MVT) and concomitant complications [2, 3]. Therefore, appropriate use of sedatives and analgesics is essential to keep critically ill patients comfortable and to prevent prolonged length of stay (LOS) in ICU. A potential solution to these difficulties in clinical practise is a protocol-based sedation and analgesia guideline with a clear definition of a sedation level goal [4]. The use of sedation and pain scales is, therefore, necessary for adequate monitoring. However, sedation and analgesia management protocols have not been uniformly adopted in ICUs around the world [5]. National differences were demonstrated in a Canadian survey in the use of protocol- and scoring-systems: 29% of the Canadian intensive care units use sedation and analgesic guidelines [6]. Interestingly, preceding international studies have shown adverse effects of sedation guidelines on MVT and LOS. On one hand, several studies demonstrated positive patient’s outcome associated with the use of sedation guidelines combined with sedation protocols. For example, Brook and colleagues [7] found a reduced MVT and LOS by the use of protocol-directed sedation on a medical ICU. Furthermore, Kress et al. [8] identified a significant reduction of MVT and LOS by daily awakening and evaluation of the depth of sedation in patients with pulmonary disease. In addition, pharmaco-economic impact of sedation and analgesic guidelines was analysed by Mascia et al. [9] with cost-effective improvements in a prospective cost-benefit study. In contrast, other examinations did not show significant reduction of MVT by the use of standardized protocols or sedation and pain scales in general or surgical ICUs [10–13].
A systematic review showed great variability in sedative-analgesic medication during therapeutic hypothermia after cardiac arrest [14]. Interestingly, standardized sedation guidelines for patients after sudden cardiac arrest are not available, and the influence of continuous quality- and process improvement in analgesia and sedation on cardiological patients has not been sufficiently evaluated, so far. The aim of this study was to investigate the effects of a therapeutic algorithm-based sedation protocol on MVT and LOS in patients after sudden cardiac arrest in a single-centre cardiological ICU.
Methods
Patients
We included 100 patients with positive outcome after sudden cardiac arrest and successful extubation after critical care therapy (Fig. 1). Patients with endotracheal intubation and MVT over 24 h were included if they were aged between 18 and 75 years. Resuscitations were 95% out of hospital, with reported resuscitation periods between 1 and 15 min. Due to the underlying diagnosis of myocardial infarction in 90 cases, PCI was performed [15]. In 5% of these patients, resuscitation was necessary due to peri-interventional arrhythmias (e.g. ventricular fibrillation) [16]. Cardiogenic shock due to heart failure was diagnosed in nine included patients and acute renal failure in one patient. Previous studies have shown positive effects of mild hypothermia in patients after sudden cardiac arrest [17, 18]. All the patients received mild therapeutic hypothermia for a maximum of 24 h with endovascular cooling conforming due to current post-cardiac-arrest-care guidelines of the American Heart Association [19]. During this period, deep sedation [Richmond Agitation Sedation Scale 4-5 (RASS 4-5)] and deep analgesia were defined for the patients in the interventional group, while in the control group, the sedation and analgesia were kept at a constant high level.
Clinical data (like MVT and LOS) were obtained from our clinical IT system. We calculated Acute Physiology and Chronic Health Evaluation II scores (APACHE II) on the basis of clinical data available from the first 24-h period of intensive care. According to Knaus et al. [20], we chose an APACHE II score cut-off of 25 distinguishing severity of illness in our specific study population. Subgroup analysis was performed to evaluate subgroups that might show a tendency towards shorter or longer MVT.
We collected demographic data like age, gender, diagnoses and comorbidity. We excluded patients with lethal exit and patients with cerebral nervous system impairment after clinical neurological examinations including EEG, evoked potential and/or cerebral imaging performed by the neurological department. Patients after cardiac arrest received no regular tracheostoma due to estimated short time ventilation. Patients who received tracheostomy were diagnosed with cerebral hypoxia or severe COPD and were transferred to external hospitals. To detect the primary endpoint of the study more accurately, the study was focused on patients without tracheostomy. Therefore, we excluded patients without successful extubation that required percutaneous tracheotomy, and patients that were transferred from external ICUs to our unit were excluded as well. Our study was performed on a cardiological ICU with a specific patient population that had an APACHE II score between 20 and 30. Patients with an APACHE II score higher than 30 were excluded from our study.
Design and setting
From January 2007 to December 2009, we compared a control group to an interventional group in terms of the primary endpoint MVT and the secondary endpoint LOS by means of a monocentric, prospective study. The analyses were performed in an adult cardiac ICU with 11 beds of the university hospital heart centre in Wuppertal, Germany. Control data acquisition was surveyed for 16 months between January 2007 and April 2008 (Fig. 2). The intervention, a new standardized sedation management was implemented in May 2008 followed by a 2-month training period to ascertain confident performance of each staff member. Data collection of the interventional group was acquired for 18 months from July 2008 until December 2009.
The control group sustained sedatives and analgesia conforming to former S2-guidelines, without standardized sedation management, whereas a standardized sedation management was implemented in the interventional group. For the control group, it was usual practise to reduce sedation and analgesia in weaning from mechanical ventilation without standardized protocols. Monitoring of depth of sedation was also not standardized and no pain scales were used.
Specialised medical staff including doctors and registered nurses exclusively works in the ICU in 24-h attendance. A total of six intensive care medical officers are allocated to the unit for 7 days in rotation for a minimum of 6 months. Nurses mainly performed evaluation and monitoring of depth of sedation and assessment of analgesia. Doctors in 24-h attendance on ICU were involved in targeting daily individual sedation goals. Furthermore, collaboration between nurses and doctors lead to decisions on the basis of the algorithm. The registered nurse to patient ratio is 1:2 for mechanically ventilated patients. Analgo-sedative drugs (sufentanil and midazolam), additional drugs, basic critical care practises, mechanical ventilation and weaning did not change during the study. Supplementary medication was not changed during the study and was applied conform to valid S2-guidelines of the German society of anaesthesiology and intensive care medicine for sedatives and analgesics in critical care therapy [21]. Additional drugs included propofol (Propofol-ratiopharm® 10 mg/ml), lorazepam (Tavor® pro injectione 2 mg/ml), haloperidol (Haldol®-janssen 5 mg/ml), clonidinhydrochlorid (Paracefan® 0.15 mg/ml), diazepam (Diazepam-ratiopharm® 10 mg/2 ml) and piritramide (Dipidolor® 7.5 mg/ml).
Intervention
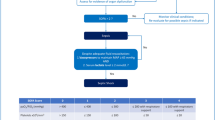
Intervention consisted of the implementation of an algorithm-directed sedation guideline according to recent guidelines and the study by Brook et al. [7]. Therefore, sedation and pain level as well as medication dosage were documented every 4 h and defined as ‘actual state’. The RASS was used for quantification of sedation [22]. We used a pain scale ranging from 1 (no pain) to 6 (intolerable pain) to quantify pain sensation. Vegetative symptoms, such as blood pressure and heart rate were incorporated in pain evaluation as well as patient’s mimic. Additional diseases (e.g. sepsis) or specific therapy (e.g. controlled hypothermia), which required particular depth of sedation were documented as well. The algorithm (Fig. 3) and RASS table were placed at every patient’s bed.
The ‘target state’ of sedation and pain level was defined daily in the morning rounds after assessing the individual therapy plan for each patient. Depending on the patient’s condition a transient deep sedation such as RASS −4 was required for hemodynamic unstable patients, whereas moderate to light sedation (RASS 0 to −1) was aimed for patients during weaning from mechanical ventilator. If the ‘actual state’ (defined by the patient’s actual status of RASS and pain level) differed from the defined ‘target state’, analgo-sedative medication was adapted according to the algorithm, with the aim of assimilation between ‘actual state’ and ‘target state’. The algorithm included instructions for ‘over-sedated’ and ‘under-sedated’ patients (Fig. 3). ‘Over-sedated’ patients were deeper sedated (mostly RASS −3 to −5) than the defined ‘target state’. ‘Under-sedated’ patients were lighter sedated or agitated (mostly RASS +2 to +4) compared to the defined ‘target state’.
Statistical analysis
All data were transferred to Excel (Microsoft, Redmond, WA, USA). Statistical analyses were performed with STATA 10.1 software (StataCorp LP, Texas, USA). Sample size calculation was based on an assumption to reduce MVT by 50% (from 175 h, SD 205.9 to 84 h, SD 100) to demonstrate a power of 80% and an α-level of 0.05. Thereby, a sample size of 100 patients was included. The comparison of the control group and interventional group concerning demographic data as age and gender distribution were analysed using Fisher’s exact test. To examine differences in location between the control and interventional data for the MVT and LOS we used non-parametrical statistics, including the Wilcoxon rank-sum test. p values less than 0.05 were considered significant. The Kaplan–Meier method was used to assess cumulative distribution function on MVT and LOS. Acute Physiology and Chronic Heath Evaluation (APACHE) II scores of all included patients were categorized and Fisher’s exact test was used to examine if there is coherence between distribution and groups. The effect of the APACHE II score on MVT and LOS as well as the total dose of midazolam and sufentanil compared between the groups was also examined using Wilcoxon rank-sum test.
Ethical regulations
The terms of the latest version of the Declaration of Helsinki for Medical Research involving human subjects have been adhered to. The ethical commission of the university Witten/Herdecke has approved this study (Reference-No. 79/2008). Patients with emergency endotracheal intubation and effective resuscitation received information from the director of the study after successful weaning and extubation. The patients or relatives gave written informed consent for the study after sufficient time for consideration and information.
Results
We examined a total of 100 patients with 45 patients for the control group and 55 patients for the interventional group. We excluded a total number of 170 patients from our study due to tracheotomy, lethal exit, extensive neurological deficiency, severe respiratory failure or sepsis (Fig. 1). Amongst these patients, 35 patients who were transferred from other hospitals were excluded to prevent possible interaction with external sedation practises. Data for patients with self-extubations and re-intubations were not subjected to statistical testing. The control group and interventional group were similar for characteristics like gender, diagnosis and comorbidity (Table 1). There was no statistical significant difference between both groups for mean midazolam and sufentanil dose, as well as mean APACHE II score, with a tendency for higher APACHE II scores in the interventional group. The total study population had a mean APACHE II score of 25.4. Age distribution of the study population showed a mean age of 58.1 ranging from 39 to 75 years. However, we found a trend towards older patients in the interventional group.
Patients that had an APACHE II score under 25 had a median MVT of 173.0 h in the control group and a median MVT of 110.0 h in the interventional group. Whereby, patients with an APACHE II score between 25 and 29 showed a significant elongation of median MVT from 92.0 to 185.0 h in the interventional group (p = 0.008; Figs. 4, 5).
For the median LOS in patients with an APACHE II score smaller than 25, we found 11.0 days in the control group versus 10.0 days in the interventional group; however, there was no statistical significance (Table 2; Figs. 6, 7). On the contrary, patients with an APACHE II score between 25 and 29 showed a significant elongation in median LOS with 6.5 days in the control group and 13.0 in the interventional group (p < 0.001). However, we found no significant difference between the complete groups for MVT and LOS (Table 2). We observed an increased communication between doctors and nursing staff especially concerning analgo-sedative therapy. However, the introduction of a new algorithm led to a more standardized management to achieve specific sedative goals.
Discussion
Analgesics and sedative medication are essential in therapy of critically ill patients especially during mechanical ventilation [1–3, 23, 24]. The present study compared non-protocol-directed with a standardized algorithm-based sedation management in mechanically ventilated patients after sudden cardiac arrest.
The current level of evidence of sedation guidelines associated with patients after sudden cardiac arrest is low and multiple underlying causes for sudden cardiac arrest are described [25–29]. A recent review analysed the critical care therapy in resuscitated patients and found 26% of the analysed 44 studies did not report any standardized analgo-sedative management [14]. The authors suggested a sedation guideline based on their analysis using propofol and remifentanil. However, this is not recommended for long-term mechanical ventilation and needs further investigation. There is a lack of additional studies revealing the influence of sedation protocols on MVT in patients after sudden cardiac arrest.
Recent studies on ventilation time during an acute myocardial infarction identified this specific collective as a high-risk group with a mortality of about 50% [23]. However, our data revealed a benefit for younger patients without severe illness by the use of sedation guidelines with a tendency to shorter MVT and LOS. Whereby, significant prolongation of MVT and LOS was observed for patients with higher APACHE II score (>25). Whereas, the median increase in MVT from 102.0 to 160.0 h between the total control and interventional group must be controversially discussed. The baseline characteristics as gender, diagnosis were similar in both groups due to strict inclusion criteria.
Correspondent to our observations, studies including patients with lower APACHE II scores showed benefits after implementation of a standardized sedation practise. In a randomized controlled trial, Brook and colleagues [7] reported substantially reduced MVT, LOS and need for tracheotomy in critically ill patients with a mean APACHE II score of 23 and acute respiratory failure. Tobar et al. [30] demonstrated a reduced dosage of midazolam after the implementation of sedation guidelines only in patients with APACHE II scores between 16 and 19. In a prospective-retrospective study Marshall and colleagues [4] showed a decreased MVT in patients with an APACHE II score between 22 and 24 receiving continuous sedation in a general ICU.
However, in our study, mean APACHE II scores ranged between 24.5 for the control and 26.4 for the interventional period, as an indicator for severe ill patients in particular for the interventional group. We, therefore, assume that severity of illness classified by APACHE II score is an important factor affecting MVT and LOS. However, a relationship between severity of illness and duration of mechanical ventilation in critically ill patients sustaining continuous sedation and analgesia has not been standardized evaluated, so far. Whereas, Wang et al. described a relationship between severity of illness and psychological adverse events in conscious ICU patients. In their multi-centre study higher APACHE II scores were associated with more invasive medical and nursing procedures [31]. In a meta-analysis of 11 studies including a total of 220,000 patients, Frost et al. [32] found a relationship between increasing intensive care severity of illness and risk of re-admission to ICU.
Similar to our results, Williams and colleagues likewise did not find a reduction of MVT in an Australian general ICU after implementation of sedation and pain scales in 2008. The authors assumed a lack of effect in the practise of scales that complement existing sedation and analgesia management in situations in which the MVT is already low compared with that of other ICUs. Interestingly, APACHE II score was shown to be an important determinant for mechanical ventilation of patients that were ventilated for 96 h or more [13]. Although study population (mean APACHE II score between 16.8 and 17.4) and median MVT (24 h pre-interventional vs. 28 h post-interventional) of this trial is not comparable to our study, lack of effect in our trial may be due to already low MVT in our specific study population.
In addition, a randomized trial of Bucknall et al. provided no reduction of MVT or LOS, by the use of protocol-directed sedation compared with usual local management. Mean age in the group of patients was 56.1 in the control group and 58.2 in the protocol group. This is similar to our age distribution with a mean age of 56.3 in the control group and 60.9 in the interventional group. Furthermore, patients with greater severity of illness showed a lower success rate in weaning from mechanical ventilation [10]. Elliott and co-workers demonstrated in a pre- and post-interventional comparative study that the use of an algorithm-based sedation guideline did not reduce MVT. Mean age of patients was likely high with 63.6 in the pre-interventional and 66.1 in the post-interventional group [11]. Since age and severity of illness are most likely related, we suggest that similar to the APACHE II score, age plays an important role in determining MVT and LOS. Therefore, another reason for lack of significant reduction of MVT and LOS in this study may be due to an unbalanced age distribution between both groups and higher APACHE II scores in the interventional group (Table 1).
Previous studies indicate low levels of monitoring. For example, a standardized management of treatment in various diseases such as blood pressure control, especially in patients at an increased risk for cardiovascular events is lacking. In this study, the importance of improved hypertension management as recommended by current treatment guidelines is emphasized [33].
Several studies on treatment of multiple diseases have shown improvement in prognostic implications by implementation of new management by optimizing and standardizing patients’ therapy [34–36]. However, comparison of results on sedation management from international studies is moreover complicated because of different therapy standards in critical care: there is a variety of basic ICU conditions (e.g. nurse-patient ratio) and of sedative and analgesic medication (e.g. lack of availability of sedative agents). Diversity in sedation practises may explain adverse results of previous studies on MVT. European standards in critical care include midazolam, morphine and fentanyl. Whereby, morphine plays a lower role in Germany concerning continuous analgesia [37]. Previous studies showing a reduction of MVT, like Brook and colleagues and the group of Marshall et al. used lorazepam/fentanyl [4, 7]. Others preferred midazolam/fentanyl or propofol like Williams and co-workers [13], who found no reduction of MVT. In a randomized clinical trial, patients receiving a midazolam infusion had statistically longer time intervals from discontinuation of drug infusion until extubation compared to patients receiving propofol infusions (97.9 ± 54.6 h vs. 34.8 ± 29.4 h; p < 0.001) [38]. The authors explained this difference in the more rapid reversal of the sedative properties of propofol compared to midazolam as it has also been suggested by other examinations as well [39]. Furthermore, inconsistency occurs in the nurse to patient ratio, which may also influence MVT and LOS. Our study was performed in a close unit with 24-h medical staff attendance with a nurse to patient ratio of generally 1:2, whereas in other studies 1:1 nurse to patient ratios are established [1, 40].
Furthermore, implementation of a new sedation management resulted in changes in medication every 4 h, whereas medication was mostly kept constant at a high level in the control group.
We collected our data from January 2007 until December 2009. Our study began before the current German S3-guidelines for the management of analgesia, sedation and delirium in intensive care were published; therefore, we performed the study in valid S2-guidelines [21, 41]. In the meantime, current S3-guidelines recommend the use of scores and protocols for the management of sedation and analgesia into routine ICU practise.
Limitations of the study
Comparison of retrospective with prospective data limits the comparability of both groups with lack of randomisation. To demonstrate consistency of groups, we correlated gender, diagnosis and applied strict inclusion criteria. An undistorted prospective collection of patients’ data was not possible due to raised awareness concerning the changed sedation practises in the medical staff. In addition, denying a standardized scheme to one of the randomised groups could have been ethically questionable. According to these reasons, a retrospective collection of control group data was necessary. Furthermore, this study was performed in a single-centre setting; therefore, the results may not be directly applicable to other ICUs caring for different groups of critically ill patients with specific sedation practises.
The study was focused on patients without tracheostomy. Therefore, we do not know if the patients with or without tracheostomy would have benefited from the new sedation management.
Furthermore, accurate details on the majority of duration of resuscitation time was hardly documented and if so periods between 1 and 15 min were documented. It has to be considered that information was mostly based on third-party anamnesis, which is potentially uncertain in such conditions. Therefore, statistical analysis would not have been reasonable.
In conclusion, MVT and secondary LOS are influenced by the severity of illness and patient’s age. Our data demonstrate the effects of sedation guidelines after sudden cardiac arrest. In particular, young cardiological patients without increased comorbidity benefit from an implementation of specific sedation guidelines. Further investigations are necessary to evaluate our results in a larger collective and to identify additional factors affecting MVT and LOS.
References
Jacobi J, Fraser GL, Coursin DB et al (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 30:119–141
Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G (1998) The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 114:541–548
Quenot JP, Ladoire S, Devoucoux F et al (2007) Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med 35:2031–2036
Marshall J, Finn CA, Theodore AC (2008) Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med 36:427–433
Sessler CN, Wilhelm W (2008) Analgesia and sedation in the intensive care unit: an overview of the issues. Crit Care (London, England) 12(Suppl 3):S1
Mehta S, Burry L, Fischer S et al (2006) Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med 34:374–380
Brook AD, Ahrens TS, Schaiff R et al (1999) Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 27:2609–2615
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342:1471–1477
Mascia MF, Koch M, Medicis JJ (2000) Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med 28:2300–2306
Bucknall TK, Manias E, Presneill JJ (2008) A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med 36:1444–1450
Elliott R, McKinley S, Aitken LM, Hendrikz J (2006) The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive Care Med 32:1506–1514
MacLaren R, Plamondon JM, Ramsay KB, Rocker GM, Patrick WD, Hall RI (2000) A prospective evaluation of empiric versus protocol-based sedation and analgesia. Pharmacotherapy 20:662–672
Williams TA, Martin S, Leslie G et al (2008) Duration of mechanical ventilation in an adult intensive care unit after introduction of sedation and pain scales. Am J Crit Care 17:349–356
Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandin B (2010) Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg 110:1328–1335
Bauer T, Hoffmann R, Jünger C et al (2009) Efficacy of a 24-h primary percutaneous coronary intervention service on outcome in patients with ST elevation myocardial infarction in clinical practice. Clin Res Cardiol 98:171–178
Tebbe U, Hochadel M, Bramlage P et al (2009) In-hospital outcomes after elective and non-elective percutaneous coronary interventions in hospitals with and without on-site cardiac surgery backup. Clin Res Cardiol 98:701–707
Jacobshagen C, Pelster T, Pax A et al (2010) Effects of mild hypothermia on hemodynamics in cardiac arrest survivors and isolated failing human myocardium. Clin Res Cardiol 99(5):267–276
Koester R, Kaehler J, Barmeyer A, et al. (2011) Coronary angiography and intervention during hypothermia can be performed safely without cardiac arrhythmia or vasospasm. Clin Res Cardiol. (doi:10.1007/s00392-011-0334-z)
ECC Committee, Subcommittees and Task Forces of the American Heart Association (2005) American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 112(24 Suppl):IV1–IV203
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Martin J, Parsch A, Franck M, Wernecke KD, Fischer M, Spies C (2005) Practice of sedation and analgesia in German intensive care units: results of a national survey. Crit care (London, England). 9:117–123
Sessler CN, Gosnell MS, Grap MJ et al (2002) The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 166:1338–1344
Kouraki K, Schneider S, Uebis R et al (2011) Characteristics and clinical outcome of 458 patients with acute myocardial infarction requiring mechanical ventilation. Results of the BEAT registry of the ALKK-study group. Clin Res Cardiol 100:235–239
Oldenburg O, Bitter T, Lehmann R et al (2011) Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol 100:107–115
Smilde TD, van Veldhuisen DJ, van den Berg MP (2009) Prognostic value of heart rate variability and ventricular arrhythmias during 13-year follow-up in patients with mild to moderate heart failure. Clin Res Cardiol 98:233–239
Dellas C, Chapuy B, Schweyer S et al (2009) A rare cause of sudden cardiac arrest: primary cardiac lymphoma. Clin Res Cardiol 98:509–511
Herren T, Gerber PA, Duru F (2009) Arrhythmogenic right ventricular cardiomyopathy/dysplasia: a not so rare “disease of the desmosome” with multiple clinical presentations. Clin Res Cardiol 98:141–158
Letsas KP, Efremidis M, Kounas SP et al (2009) Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol 98(4):208–212
Huntgeburth M, Laudes M, Burst V, Krämer S, Reuter H, Rosenkranz S (2011) Acute cardiac arrest secondary to severe hyperkalemia due to autoimmune polyendocrine syndrome type II. Clin Res Cardiol 100:379–382
Tobar E, Bugedo G, Andresen M et al (2009) Characteristics and impact of sedation, analgesia, and neuromuscular blockage in critical patients undergoing prolonged mechanical ventilation. Med Intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias 33:311–320
Wang Y, Ma PL, Liu JT, Su JW, Li Q, Zeng L (2008) Relationship between severity of illness and mental status in ICU conscious patients: a nationwide multi-center clinical study. Zhonghua Yi Xue Za Zhi 88:1450–1453
Frost SA, Alexandrou E, Bogdanovski T et al (2009) Severity of illness and risk of readmission to intensive care: a meta-analysis. Resuscitation 80:505–510
Thoenes M, Tebbe U, Rosin L et al (2011) Blood pressure management in a cohort of hypertensive patients in Germany treated by cardiologists. Clin Res Cardiol 100:483–491
Keller T, Post F, Tzikas S et al (2010) Improved outcome in acute coronary syndrome by establishing a chest pain unit. Clin Res Cardiol 99:149–155
Gerckens U, Latsios G, Mueller R et al (2009) Left main PCI after trans-subclavian CoreValve implantation. Successful outcome of a combined procedure for management of a rare complication. Clin Res Cardiol 98:687–690
Birkemeyer R, Rillig A, Koch A et al (2010) Primary angioplasty for any patient with ST-elevation myocardial infarction? Guideline-adherent feasibility and impact on mortality in a rural infarction network. Clin Res Cardiol 99:833–840
Soliman HM, Melot C, Vincent JL (2001) Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth 87:186–192
Barrientos-Vega R, Mar Sanchez-Soria M, Morales-Garcia C, Robas-Gomez A, Cuena-Boy R, Ayensa-Rincon A (1997) Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med 25:33–40
Carrasco G, Molina R, Costa J, Soler JM, Cabre L (1993) Propofol vs midazolam in short-, medium-, and long-term sedation of critically ill patients. A cost-benefit analysis. Chest 103(2):557–564
Tallgren M, Pettila V, Hynninen M (2006) Quality assessment of sedation in intensive care. Acta Anaesthesiol Scand 50:942–946
Martin J, Heymann A, Bäsell K et al (2010) Evidence and consensus-based German guidelines for the management of analgesia, sedation and delirium in intensive care—short version. Ger Med Sci 8:Doc 02 (PMID 20200655)
Acknowledgments
The study was funded by HELIOS Research Center GmbH, Friedrichstraße 136, 10117 Berlin, Germany (Funding ID 2041).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abanador-Kamper, N., Kamper, L., Wolfertz, J. et al. Influence of algorithm-based analgesia and sedation in patients after sudden cardiac arrest. Clin Res Cardiol 101, 175–183 (2012). https://doi.org/10.1007/s00392-011-0378-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-011-0378-0