Abstract
Purpose
This single-centre cohort study was designed to identify factors that can predict primary tumour downstaging by neoadjuvant chemoradiotherapy (nCRT) in rectal carcinoma.
Methods
Prospectively collected data from 555 patients with clinical T category (cT) cT3-4 rectal carcinoma treated between 1995 and 2019 were retrospectively analysed. All patients received long-term neoadjuvant chemoradiotherapy followed by surgery with curative intent at the Department of Surgery, University Hospital Erlangen, Germany. Patient-, tumour- and treatment-related factors with a potential impact on the downstaging of rectal carcinoma to pathological T category (pT) ≤ ypT2 and ypT0 were analysed in univariate and multivariate logistic regression analyses. The prognosis of patients with and without downstaging of the primary tumour was compared.
Results
A total of 288 (51.9%) patients showed downstaging to ≤ ypT2. Eighty-six (15.5%) patients achieved clinical complete regression (ypT0). In the multivariate logistic regression analysis, the factors cT category, BMI, ECOG score, CEA, histological type, extension in the rectum and year of the start of treatment were found to be independent factors for predicting downstaging to ≤ ypT2 after neoadjuvant chemoradiotherapy. The year of treatment initiation also remained an independent significant predictor for pathological complete regression. The prognosis was superior in patients with downstaging to ≤ ypT2 in terms of locoregional and distant recurrence as well as disease-free and overall survival.
Conclusion
Factors predicting downstaging after long-term nCRT could be identified. This may be helpful for counselling patients and selecting the optimal treatment for patients with advanced rectal carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is one of the most common cancers worldwide and was responsible for nearly 10% of cancer deaths in 2020 [1]. In recent decades, prognosis has been significantly improved by two important factors: the total mesorectal excision (TME) technique and radio(chemo)therapy for patients with advanced rectal carcinoma. As a result of both, a significant reduction in locoregional recurrence rates has been observed.
The concept of total mesorectal excision (TME) for rectal cancer was introduced by R. J. Heald [2]. With this technique, potential tumour deposits in lymph nodes and tumour cells within the mesorectum are completely removed en bloc with the tumour. The risk of local tumour recurrence can thus be lowered considerably [2,3,4].
In the multimodal therapy of advanced rectal cancer, neoadjuvant radiation represents an important component of the treatment. In short-course radiotherapy, patients are irradiated with 5 Gy for 5 consecutive days followed by surgery within 1 week after completion. In neoadjuvant long-term chemoradiotherapy (nCRT), radiation is administered for 6 weeks with concomitant chemotherapy during the first and fifth week, and surgery is performed six to eight weeks after the end of radiation. During this period, regression of the tumour can be expected. The CAO/ARO/AIO-94 trial has shown that preoperative chemoradiotherapy leads to better local control and is associated with lower toxicity than postoperative chemoradiotherapy [5,6,7]. Since then, neoadjuvant chemoradiotherapy followed by TME has become the standard treatment for locally advanced rectal cancer (cT3-4 or cN +) in many countries, including Germany.
The success of neoadjuvant therapy is mainly described in the histopathological examination of the resected specimen with downstaging from the clinical pretherapeutic cT category to the pathological ypT category after chemoradiation. Due to the lower sensitivity and specificity of the clinical N category, the corresponding downstaging of the regional lymph node status from cN + to ypN0 is less appropriate to describe this success. Furthermore, a reduction in tumour cells can be described and classified according to regression systems such as that of Dworak [8].
Neoadjuvant long-term chemoradiotherapy, including the subsequent consolidation phase, lasts at least 12 weeks until the carcinoma is finally removed. It is therefore important to know which patients will particularly benefit from this treatment. The aim of this study was to investigate which patient-, tumour- and treatment-related factors have an impact on the downstaging of rectal carcinoma. The primary endpoint of this study was downstaging from cT3-4 to a ypT category ≤ ypT2, and the secondary endpoint was downstaging of rectal carcinoma to ypT0 (pathological complete regression).
Methods
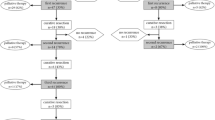
This single-centre cohort study included a total of 567 patients with primary rectal carcinoma (cT3-4 any cN M0) who underwent neoadjuvant long-course chemoradiotherapy (nCRT) followed by surgery with curative intent (R0/R1) at the Department of Surgery, University Hospital Erlangen, Germany, between 1995 and 2019. Patients were selected based on the following inclusion criteria: solitary invasive rectal carcinoma with a distal margin < 12 cm from the anal verge, measured with a rigid sigmoidoscope; carcinoma not associated with familial polyposis, ulcerative colitis or Crohn´s disease; and pretherapeutic staging cT3-4 any cN M0. Patients with other synchronous or metachronous malignancies and patients with clinical (nearly) complete remission on a ‘watch and wait’ strategy after nCRT were excluded. One patient with a rectal perforation 10 days after nCRT and therefore premature tumour resection was excluded, as were 11 patients with delayed resection after an extended interval between the end of nCRT and surgery of more than 6 months. Overall, 555 patients were analysed.
General epidemiological data, clinical findings and treatment as well as histopathological findings were collected prospectively at the Erlangen Registry for Colorectal Carcinomas (ERCRC). Retrospectively, additional potential predictive factors for downstaging were assessed.
Prior to the start of nCRT, all patients underwent thorough preoperative diagnostics and staging. In accordance with the respective current German S3 guideline [9], the following examinations were performed: rectoscopy with biopsy, abdominopelvic computed tomography (CT) and/or, from 2005, increasingly magnetic resonance imaging (MRI) to assess the depth of tumour invasion, lymph node status and involvement of the mesorectal fascia (MRF), chest X-ray/CT and serum tumour markers (CEA, CA19-9).
All patients underwent long-course nCRT. Radiotherapy was given to a cumulative dose of 50.4 Gy (28 fractions of 1.8 Gy, 5 days per week) to the pelvis. Chemotherapy was given either in the first and fifth week with infusional 5-FU 1000 mg/m2 d1-5 or 5-FU 250 mg/m2 d1-14,21–34 plus oxaliplatin 50 mg/m2 d1,8,21,29. In a few patients, oxaliplatin was replaced by irinotecan or irinotecan monotherapy. The selection of chemotherapeutic agents depended on whether the patients were treated at the centre, at their local hospital or within clinical trials. Ninety-six of the patients received hyperthermia in an ongoing study. This involved heating the tumour tissue to 40 to 43°C for approximately an hour, once or twice a week for up to 10 sessions.
Six to eight weeks after completion of chemoradiotherapy, TME surgery was performed with precise dissection within the visceral and parietal fascia sparing the autonomic nerve structures whenever possible and appropriate. The final decision on the surgical method, in particular sphincter preservation yes or no, was always made intraoperatively.
The detailed documentation of the histopathological findings enabled the classification of the carcinomas according to the 8th edition of the TNM classification of the UICC [10]. A tumour that has invaded the perirectal fat tissue is classified as T3, and a tumour with perforation of the visceral peritoneum or invasion into other organs or structures is classified as T4.
Tumour downstaging was defined according to Yoon [11] as histopathologic downstaging of the primary tumour from cT3-4 to ypT2 or less. The Dworak grading system with a scale from 0 to 4 was used to determine histopathological tumour regression [8]. Grade 4 was defined as pathological complete regression corresponding to ypT0. Pathological complete regression (pCR) is the absolute absence of tumour cells in the resected specimen.
Body mass index (BMI) was classified according to the definition of the World Health Organization (WHO): underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥ 30.0 kg/m2) [12].
Patients were followed up for at least 5 years, in the first 2 years at 3-month intervals and thereafter at 6-month intervals. In 2004, the follow-up interval was changed according to the guidelines: every 6 months for the first 2 years and annually thereafter. Follow-up data were collected either through follow-up visits at the university hospital or through written correspondence with the patients’ treating physicians. After 5 years, at least a vital status check was carried out annually at the local registration office.
Statistical analysis
To compare categorical data, the χ2 test was used, and Fisher’s exact test was used for values < 5. The Mann–Whitney U test was applied for quantitative data. Univariate logistic regression analyses were used to assess the interaction of potential predictive factors on downstaging of the T category. Variables that reached significance at p < 0.05 in the univariate analysis were included in a multivariate model. As the distance of the carcinoma to the mesorectal fascia (MRF) was determined in less than 50% of the patients (n = 259), this factor was not included in the multivariate analysis.
For the analysis of prognosis, only patients treated between 1995 and 2015 with a potential follow-up of at least 5 years were considered. Two patients with missing follow-up information were excluded; thus, 471 patients were evaluated. Disease-free survival (DFS) was defined as the time interval from primary surgery to the occurrence of local recurrence, distant metastasis or death by any cause. For overall survival (OS), the patient’s death from any cause was determined as the endpoint. Survival was estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. The 95% confidence intervals (95% CI) were calculated according to Greenwood [13]. A two-sided p value < 0.05 indicated a significant difference. All analyses were performed with the statistical software IBM SPSS® version 28 (IBM, Armonk, NY, USA).
Results
The patients’ tumour and treatment characteristics are summarised in Table 1. At a median of 7.3 weeks (IQR 6.7–8.6) after the completion of neoadjuvant chemoradiotherapy, TME surgery was performed.
The distribution of pathologic tumour response in terms of ypT category and Dworak regression grade is illustrated in Table 2. A total of 288 (51.9%) patients presented with downstaging to ypT2 or less after neoadjuvant chemoradiotherapy. Eighty-six (15.5%) were classified as ypT0 as pathological complete regression (Dworak grade 4).
Patient-related predictive factors associated with a significantly higher frequency of downstaging to ≤ ypT2 were as follows: a favourable ECOG performance score 0–1 (54% vs 20%; p < 0.001), a regular CEA level < 5 ng/ml (58% vs 35%; p < 0.001), a low CRP value < 5 mg/l (60% vs 35–52%; p = 0.018) and a normal weight or overweight body mass index (BMI) (55–58% vs 23–40%; p = 0.002, Table 3).
Tumour-related predictive factors and their association with downstaging are presented in Table 4. Downstaging was observed significantly more frequently in carcinomas with the following characteristics: cT3 (56% vs 36%; p < 0.001), insular or semicircular extension in the rectum (55–65% vs 43%; p < 0.001), adenocarcinoma as the histological type (53% vs 22%; p = 0.002) and distance to mesorectal fascia (MRF) > 1 mm (64% vs 48%; p = 0.037).
The year of treatment initiation showed a significant improvement in downstaging (Table 5). Carcinomas from patients treated between 2011 and 2019 were diagnosed more frequently as ≤ ypT2 than previously treated patients (56% vs 40 and 54%; p = 0.025).
With regard to pathological complete regression (ypT0), no patient- or tumour-related predictive factors reached the level of significance. However, over the years, the complete regression increased from 7.5 to 18.2% (p = 0.035). Thus, the year of treatment was confirmed as a significant predictive (treatment-related) factor.
Potential blood value-related predictive factors for downstaging to ≤ pT2 or ypT0 are listed in Supplementary Table 1. Elevated neutrophil granulocytes were significantly less frequently associated with a pathological complete response.
In the multivariate logistic regression analysis, the factors cT category, BMI, ECOG score, CEA, histological type, extension in the rectum and the year of the start of the treatment were found to be independent factors for predicting downstaging to ≤ ypT2. In addition, the year of treatment initiation remained an independent significant predictor for pathological complete regression (ypT0; Table 6).
Prognosis
For the analysis of prognosis, 239 patients with downstaging to ≤ ypT2 after chemoradiotherapy were compared to 232 patients without downstaging (ypT3-4). In all analyses, for locoregional and distant recurrences as well as for disease-free survival (DFS) and overall survival (OS), the prognosis was superior in patients with downstaging to ≤ ypT2 (p < 0.001 each; Table 7, Fig. 1). For locoregional recurrence, the difference was 9 percentage points within 5 years (2.2% vs 11.8%). For distant metastasis, a threefold increase was observed in patients without downstaging (11.9% vs 33.6%). This resulted in a difference of 34 percentage points in DFS (93.2% vs 59.0%) and 16 percentage points in OS (89.9% vs 73.6%).
This was also evident when comparing the 64 patients with pathological complete regression (ypT0) with the 407 patients diagnosed with ypT1-4. Again, there was a clear difference with a significantly better prognosis in the patients with ypT0. No patient with pathological complete regression had developed locoregional recurrence within 5 years (p = 0.014), and only one patient experienced distant metastasis after 26 months (p < 0.001). For patients with ypT0, DFS improved by 30 percentage points (96.9% vs 67.3%) and OS by 18 percentage points (98.4% vs 79.3%; p < 0.001 each).
Discussion
Neoadjuvant chemoradiotherapy (nCRT) has significantly improved the prognosis of patients with advanced rectal cancer, in particular by reducing the local recurrence rate. However, there is an ongoing debate about the indication for nCRT, i.e. which patients would actually benefit from nCRT and expect a good response. In this study, we identified several factors in multivariate analysis that may predict downstaging from advanced rectal carcinomas (cT3-4) to ≤ ypT2. We found several patient-related independent predictive factors, such as BMI, ECOG score and CEA; tumour-related predictive factors, such as cT category, extension in the rectum and histological type; and one treatment-related factor reflecting the year of treatment. The latter also proved to be an independent predictive factor for a pathological complete response (ypT0).
Patient-related predictive factors
In this study, BMI was proven to be an independent predictive factor for downstaging. Normal weight patients experienced significantly better downstaging ≤ ypT2 of the primary tumour than underweight (< 18.5 kg/m2) and obese (≥ 30.0 kg/m2) patients. This is confirmed by data from Sun et al. and Ottaiano et al., who found that obesity leads to poorer downstaging of the T category and more frequent side effects, which may result in lower doses of chemotherapy [14, 15]. In patients with thoracic tumours, Zhao et al. found an association between increased BMI and raised so-called setup errors at daily positioning for radiation [16].
The ECOG performance score describes the general condition of oncological patients with regard to their functional abilities to care for themselves, their daily activity and ability to work and their need for care [17]. In this study, the ECOG score was identified as an independent predictive factor for downstaging in the multivariate analysis. To our knowledge, this factor has not been investigated in other studies on rectal cancer.
Human carcinoembryonic antigen (CEA) is a broadly used tumour marker recognised for staging, disease surveillance and follow-up. In this study, the pretherapeutic CEA level provides a prediction of the response to nCRT. This is consistent with the results of the studies of Yoon et al. [11], Das et al. [18] and Park et al. [19]. Patients with an elevated CEA level responded worse to nCRT than patients with a regular value. The CEA level was found to be not only a predictive factor for downstaging but also a predictor for pathological complete regression. Ordonez et al. explained the influence of CEA by the fact that CEA inhibits cell apoptosis. Tumour cells that overexpress CEA are therefore resistant to nCRT [20].
Tumour-related predictive factors
The clinical T category was also confirmed in this study as an independent predictive factor for downstaging ≤ ypT2. This is to be expected, as cT3 carcinomas invade only into the perirectal fat tissue, whereas cT4 carcinomas infiltrate beyond, into visceral peritoneum or neighbouring organs or structures. This is consistent with the results of Yoon et al. [11], who also showed that cT3 is a predictor of advanced tumour downstaging. Zhang et al. [21] demonstrated a strong correlation between the cT category and pathological complete regression of rectal carcinoma. The clinical N category is not used as a benchmark for downstaging because of its low sensitivity and specificity.
Most rectal carcinomas in our study presented as conventional adenocarcinoma (95%). The few mucinous and signet ring carcinomas responded significantly worse to nCRT. This is controversial in the literature. Engineer et al. [22] also described rare downstaging in patients with signet ring cell carcinoma, and McCawley et al. [23] described both a lower rate of downstaging and pathological complete regression in mucinous carcinoma. Jayanand et al. [24] found that the pathological complete regression rate is higher in signet ring cell carcinoma.
The extent of carcinoma in the rectum was identified as another significant predictive factor. An insular noncircular extension resulted in a better response and thus better downstaging. The study by Jayanand et al. [24] also revealed that pathological complete regression was achieved more frequently with noncircumferential extension. This result was also confirmed by Das et al. [18] for both downstaging and pathological complete regression. Subsequently, a circular extension of the carcinoma is associated with a worse prognosis [25, 26].
The distance to the mesorectal fascia on imaging is a relatively new but important factor for indication and prognosis in rectal cancer. As a possible predictive factor, it had to be excluded from our multivariate analysis because of too many missing values. The univariate analysis indicated that it is a predictive factor for downstaging. Ren et al. [27] already proved that the distance of the tumour to the mesorectal fascia significantly influences the chance of pathological complete regression.
Treatment-related predictive factors
The year of treatment also proved to be a significant factor for downstaging. This could be due to continuous changes and thus improvements in radiation therapy and chemotherapy application. In particular, the use of moderate doses of oxaliplatin led to increased remission, measurable in the frequency of pathological complete remission [28]. In addition, the conversion from 3D radiation techniques to volumetric modulated arc therapy (VMAT) irradiation led to significantly less toxicity of radiation therapy [29]. As a result, it is more often possible to treat patients without an irradiation break, which usually leads to fewer local recurrences [30].
Prognostic factors
In this study, we identified a variety of predictive factors that are already known as prognostic factors. Predictive factors refer to factors related to response or nonresponse to a specific therapy; in this study, the downstaging of the primary tumour ≤ ypT2. Prognostic factors, on the other hand, are factors related to prognosis, i.e. disease-free survival and overall survival [31, 32].
The anatomical extent of the disease at the start of treatment (TNM) and after surgical treatment (R-classification) has been identified as the most important prognostic factor in solid tumours [33,34,35]. In this study, the cT category proved to be a powerful independent predictive factor for downstaging and a prognostic factor. Similarly, the histological type and the distance of the tumour from the mesorectal fascia are also important tumour-related prognostic factors in rectal carcinoma.
Among patient-related factors, CEA proved to be a predictive factor and has already been considered a probable essential prognostic factor by the UICC in 2006 [36] and is well supported by the literature [32]. Of particular interest is an elevated BMI, which is first a risk factor for developing colorectal cancer [37,38,39], second also a predictive factor for downstaging and third a prognostic factor [40,41,42].
Nonoperative management (NOM) - watch and wait (W&W) - total neoadjuvant therapy (TNT)
The secondary endpoint of our study was advanced downstaging to ypT0, i.e. pathological complete regression. These patients showed an excellent prognosis with an observed 5-year survival rate of 98.4%. New alternative treatment concepts named ‘nonoperative management’, ‘watch and wait’ or ‘total neoadjuvant therapy’ focus on giving patients with clinically complete remission (without pathological confirmation) only close follow-up without requiring surgery. However, this requires that the endoscopic and radiological assessment of tumour response to nCRT correlates with pathological tumour response[43]. In our department, W&W was not systematically followed. During the long study period from 1995 to 2019, the time interval after nCRT was increasingly extended from 6 to 8 weeks, especially to enable sphincter preservation in individual patients with very low rectal cancer. Finally, in the case of clinical complete remission, a W&W strategy was applied in 36 patients. While 27 patients never required surgery, nine of these patients had to undergo surgical tumour resection after 3 to 41 months.
Patients who do not require surgery are considered to have a better quality of life than patients after low anterior resection and an especially better quality of life than after abdominoperineal excision. However, even after sphincter-preserving surgery, patients may experience a variety of symptoms, such as urgency and frequency of bowel movements, bowel fragmentation, faecal incontinence or abdominal pain, collectively known as ‘low anterior resection syndrome’. Nevertheless, the consequences of chemoradiotherapy must not be disregarded [44].
Another problem is patients in whom complete regression is not achieved despite a longer waiting time after nCRT. After approximately 10 weeks, the optimal time window for surgery has passed. Inflammation, edema and fibrosis of surrounding tissues in the pelvis may have increased over time, potentially resulting in more difficult preparation, prolonged surgery time and higher morbidity [45].
Knowledge of predictive factors for downstaging in rectal cancer may be helpful to offer nCRT/TNT to those patients who are most likely to benefit and to perform primary surgery on those patients who would not benefit from nCRT/TNT.
The strengths of our study are the completeness of the prospectively collected data and a long follow-up time. The limitations of the study refer to the retrospective nature of the study and the single-centre design. In particular, the lack of data on the distance of the tumour from the mesorectal fascia due to the time period chosen limits our study.
References
WHO International Agency for Research on Cancer (2020) The Global Cancer Observatory cancer fact sheets. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed 26 January 2022
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg 69:613–616. https://doi.org/10.1002/bjs.1800691019
Bülow S, Christensen IJ, Harling H, Kronborg O, Fenger C, Nielsen HJ (2003) Recurrence and survival after mesorectal excision for rectal cancer. Br J Surg 90:974–980. https://doi.org/10.1002/bjs.4137
Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1:1479–1482. https://doi.org/10.1016/s0140-6736(86)91510-2
Sauer R, Fietkau R, Wittekind C, Rödel C, Martus P, Hohenberger W et al (2003) Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 5:406–415. https://doi.org/10.1046/j.1463-1318.2003.00509.x
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740. https://doi.org/10.1056/NEJMoa040694
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933. https://doi.org/10.1200/jco.2011.40.1836
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12:19–23. https://doi.org/10.1007/s003840050072
German Guideline Program in Oncology (German Cancer Society GCA, AWMF) (2019) S3-Guideline Colorectal Cancer, long version 2.1, AWMF registrationnumber: 021–007OL. http://www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/. Accessed 03 January 2022
UICC (International Union of Cancer) (2017) TNM Classification of malignant tumours 8th edn, Brierley JD, Gospodarowicz MK, Wittekind, Ch. (eds). Wiley Blackwell, Oxford
Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS et al (2007) Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 69:1167–1172. https://doi.org/10.1016/j.ijrobp.2007.04.047
WHO Regional Office for Europe Body mass index - BMI. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed 10 February 2022
Greenwood M (1926) A Report on the Natural Duration of Cancer. Report on public health and medical subjects. H.M, Stationery Office, London
Ottaiano A, Nappi A, Tafuto S, Nasti G, De Divitiis C, Romano C et al (2016) Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with capecitabine and oxaliplatin adjuvant chemotherapy. Oncology 90:36–42. https://doi.org/10.1159/000442527
Sun Y, Xu Z, Lin H, Lu X, Huang Y, Huang S et al (2017) Impact of body mass index on treatment outcome of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur J Surg Oncol 43:1828–1834. https://doi.org/10.1016/j.ejso.2017.07.022
Zhao J, Zhang M, Zhai F, Wang H, Li X (2018) Setup errors in radiation therapy for thoracic tumor patients of different body mass index. J Appl Clin Med Phys 19:27–31. https://doi.org/10.1002/acm2.12270
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA et al (2007) Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 109:1750–1755. https://doi.org/10.1002/cncr.22625
Park YA, Sohn SK, Seong J, Baik SH, Lee KY, Kim NK et al (2006) Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol 93:145–150. https://doi.org/10.1002/jso.20320
Ordoñez C, Screaton RA, Ilantzis C, Stanners CP (2000) Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 60:3419–3424
Zhang Y, Yan L, Wu Y, Xu M, Liu X, Guan G (2020) Worse treatment response to neoadjuvant chemoradiotherapy in young patients with locally advanced rectal cancer. BMC Cancer 20:854. https://doi.org/10.1186/s12885-020-07359-2
Engineer R, Basu T, Chopra S, Arya S, Patil P, Mehta S et al (2015) Factors influencing response to neoadjuvant chemoradiation and outcomes in rectal cancer patients: tertiary Indian cancer hospital experience. J Gastrointest Oncol 6:155–164. https://doi.org/10.3978/j.issn.2078-6891.2014.111
McCawley N, Clancy C, O’Neill BD, Deasy J, McNamara DA, Burke JP (2016) Mucinous rectal adenocarcinoma is associated with a poor response to neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Dis Colon Rectum 59:1200–1208. https://doi.org/10.1097/dcr.0000000000000635
Jayanand SB, Seshadri RA, Tapkire R (2011) Signet ring cell histology and non-circumferential tumors predict pathological complete response following neoadjuvant chemoradiation in rectal cancers. Int J Colorectal Dis 26:23–27. https://doi.org/10.1007/s00384-010-1082-7
Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM et al (2006) Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol 29:219–224. https://doi.org/10.1097/01.coc.0000214930.78200.4a
Lee SH, Hernandez de Anda E, Finne CO, Madoff RD, Garcia-Aguilar J (2005) The effect of circumferential tumor location in clinical outcomes of rectal cancer patients treated with total mesorectal excision. Dis Colon Rectum 48:2249–2257. https://doi.org/10.1007/s10350-005-0186-6
Ren DL, Li J, Yu HC, Peng SY, Lin WD, Wang XL et al (2019) Nomograms for predicting pathological response to neoadjuvant treatments in patients with rectal cancer. World J Gastroenterol 25:118–137. https://doi.org/10.3748/wjg.v25.i1.118
Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D et al (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16:979–989. https://doi.org/10.1016/S1470-2045(15)00159-X
Wee CW, Kang HC, Wu HG, Chie EK, Choi N, Park JM et al (2018) Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in rectal cancer treated with neoadjuvant concurrent chemoradiation: a meta-analysis and pooled-analysis of acute toxicity. Jpn J Clin Oncol 48:458–466. https://doi.org/10.1093/jjco/hyy029
Fietkau R, Rodel C, Hohenberger W, Raab R, Hess C, Liersch T et al (2007) Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys 67:1008–1019. https://doi.org/10.1016/j.ijrobp.2006.10.020
Clark GM (2001) Interpreting and integrating risk factors for patients with primary breast cancer. J Natl Cancer Inst Monogr:17–21. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003455
Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP (2000) American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 88:1739–1757. https://doi.org/10.1002/(sici)1097-0142(20000401)88:7%3c1739::aid-cncr30%3e3.0.co;2-t
Guerra A, Borda F, Javier Jiménez F, Martinez-Peñuela JM, Larrínaga B (1998) Multivariate analysis of prognostic factors in resected colorectal cancer: a new prognostic index. Eur J Gastroenterol Hepatol 10:51–58. https://doi.org/10.1097/00042737-199801000-00010
Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F (1998) Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum 41:1033–1049. https://doi.org/10.1007/bf02237397
Wolters U, Stützer H, Keller HW, Schröder U, Pichlmaier H (1996) Colorectal cancer–a multivariate analysis of prognostic factors. Eur J Surg Oncol 22:592–597. https://doi.org/10.1016/s0748-7983(96)92320-3
UICC (International Union Against Cancer) (2006) Prognostic Factors in Cancer, 3rd edn. Gospodarowicz MK, O'Sullivan B, Sobin LH (eds). Wiley & Sons, New Jersey
Larsson SC, Wolk A (2007) Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 86:556–565. https://doi.org/10.1093/ajcn/86.3.556
Moghaddam AA, Woodward M, Huxley R (2007) Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 16:2533–2547. https://doi.org/10.1158/1055-9965.Epi-07-0708
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578. https://doi.org/10.1016/s0140-6736(08)60269-x
Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM (2012) Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 30:42–52. https://doi.org/10.1200/jco.2011.38.0287
Kalb M, Langheinrich MC, Merkel S, Krautz C, Brunner M, Bénard A et al (2019) Influence of body mass index on long-term outcome in patients with rectal cancer-a single centre experience. Cancers (Basel) 11. https://doi.org/10.3390/cancers11050609
Lee J, Meyerhardt JA, Giovannucci E, Jeon JY (2015) Association between body mass index and prognosis of colorectal cancer: a meta-analysis of prospective cohort studies. PLoS ONE 10:e0120706. https://doi.org/10.1371/journal.pone.0120706
Greer JB, Hawkins AT (2019) Non-operative management of rectal cancer. Semin Colon Rectal Surg 30:79–84. https://doi.org/10.1053/j.scrs.2019.04.007
Lange MM, den Dulk M, Bossema ER, Maas CP, Peeters KC, Rutten HJ et al (2007) Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg 94:1278–1284. https://doi.org/10.1002/bjs.5819
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C et al (2016) Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol 34:3773–3780. https://doi.org/10.1200/JCO.2016.67.6049
Acknowledgements
The present work was performed in fulfilment of the requirements for obtaining the degree ‘Dr. med.’ at the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Clinical Ethics Committee of the Friedrich-Alexander-Universität (FAU) Erlangen-Nürnberg (132_20 Bc). According to the ethics committee, written consent was not necessary for this retrospective analysis.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kohl, V.K.B., Weber, K., Brunner, M. et al. Factors influencing downstaging after neoadjuvant long-course chemoradiotherapy in rectal carcinoma. Int J Colorectal Dis 37, 1355–1365 (2022). https://doi.org/10.1007/s00384-022-04174-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04174-y