Abstract
Purpose
The aim of this phase II study was to evaluate the efficacy and safety of combination therapy with five-cycle CAPOX (capecitabine plus oxaliplatin) plus bevacizumab, followed by five-cycle maintenance therapy with capecitabine plus bevacizumab and reintroduction of CAPOX plus bevacizumab for five cycles, with a preplanned intermittent oxaliplatin strategy in metastatic colorectal cancer (mCRC).
Methods
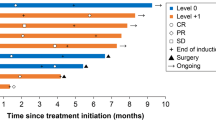
Patients with untreated mCRC were administered CAPOX (130 mg/m2 oxaliplatin on day 1, 2000 mg/m2/day capecitabine on days 1–14, every 21 days) + bevacizumab (7.5 mg/kg) every 3 weeks for five cycles, maintenance treatment without oxaliplatin for five cycles, and CAPOX + bevacizumab reintroduction for five cycles or upon tumor progression. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were the time to treatment failure (TTF), overall survival, response rate (RR), and safety.
Results
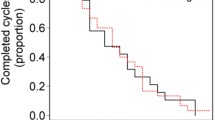
Forty-seven patients who fulfilled the inclusion criteria were enrolled in the evaluation of efficacy and safety. Median PFS was 14.1 months (95% confidence interval [CI], 8.6–19.5), and median TTF was 12.3 months (95% CI, 10.3–14.3). The objective RRs were 51.1% (24/47) during induction therapy, 58.3% (21/36) during maintenance therapy, and 63.6% (14/22) during reintroduction therapy. The frequency of patients with neutropenia, diarrhea, peripheral sensory neuropathy, venous thromboembolism, or grade ≥ 3 allergic reactions was 2.1%.
Conclusion
CAPOX plus bevacizumab therapy with a preplanned intermittent oxaliplatin strategy consisting of brief five-cycle induction therapy, five-cycle maintenance therapy with capecitabine plus bevacizumab, and five-cycle reintroduction therapy consisting of CAPOX plus bevacizumab is safe and effective for mCRC patients.
Trial registration
UMIN ID: 000,005,732, date of registration: June 7, 2011. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000006695


Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Petrelli N, Herrera L, Rustum Y et al (1987) A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol 5:1559–1565
de Gramont A, Bosset JF, Milan C et al (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15:808–815
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Cheeseman SL, Joel SP, Chester JD et al (2002) A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer 87:393–399
Goldberg RM, Sargent DJ, Morton RF et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Douillard JY, Cunningham D, Roth AD et al (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041–1047
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Cremolini C, Loupakis F, Antoniotti C et al (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16:1306–1315
Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S (2005) Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 23:3706–3712
Saltz LB, Clarke S, Diaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Martoni AA, Pinto C, Di Fabio F et al (2006) Capecitabine plus oxaliplatin (xelox) versus protracted 5-fluorouracil venous infusion plus oxaliplatin (pvifox) as first-line treatment in advanced colorectal cancer: a GOAM phase II randomized study (FOCA trial). Eur J Cancer 42:3161–3168
Cassidy J, Clarke S, Díaz-Rubio E et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26:2006–2012
Hochster HS, Hart LL, Ramanathan RK et al (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol 26:3523–3529
National Comprehensive Cancer Network (2011) NCCN Guidelines for Colon Cancer. https://www.nccn.org/professionals/physician_gls/default.aspx#colon. Accessed 6 Jun 2011
Schmoll HJ, Van Cutsem E, Stein A et al (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23:2479–2516
Watanabe T, Itabashi M, Shimada Y et al (2012) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 17:1–29
Goldberg RM, Sargent DJ, Morton RF et al (2006) Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol 24:3347–3353
Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP (2008) A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev 34:368–377
Argyriou AA, Velasco R, Briani C et al (2012) Peripheral neurotoxicity of oxaliplatin in combination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): a prospective evaluation of 150 colorectal cancer patients. Ann Oncol 23:3116–3122
Tournigand C, Cervantes A, Figer A et al (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer–a GERCOR study. J Clin Oncol 24:394–400
Chibaudel B, Maindrault-Goebel F, Lledo G et al (2009) Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 study. J Clin Oncol 27:5727–5733
Adams RA, Meade AM, Seymour MT et al (2011) Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 12:642–653
Nakayama G, Kodera Y, Yokoyama H et al (2011) Modified FOLFOX6 with oxaliplatin stop-and-go strategy and oral S-1 maintenance therapy in advanced colorectal cancer: CCOG-0704 study. Int J Clin Oncol 16:506–511
Tezuka T, Hamada C, Ishida H et al (2013) Phase II clinical study of modified FOLFOX7 (intermittent oxaliplatin administration) plus bevacizumab in patients with unresectable metastatic colorectal cancer-CRAFT study. Invest New Drugs 5:1321–1329
Díaz-Rubio E, Gómez-España A, Massutí B et al (2012) First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 17:15–25
Simkens LH, van Tinteren H, May A et al (2015) Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 385:1843–1852
Nakayama G, Ishigure K, Yokoyama H et al (2017) The efficacy and safety of CapeOX plus bevacizumab therapy followed by capecitabine plus bevacizumab maintenance therapy in patients with metastatic colorectal cancer: a multi-center, single-arm, phase II study (CCOG-0902). BMC Cancer 17:243
National Cancer Institute (2011) National Cancer Institute Common Toxicity Criteria, version 4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 6 Jun 2011
Lersch C, Schmelz R, Eckel F et al (2002) Prevention of oxaliplatin-induced peripheral sensory neuropathy by carbamazepine in patients with advanced colorectal cancer. Clin Colorectal Cancer 2:54–58
Levi F, Perpoint B, Garufi C et al (1993) Oxaliplatin activity against metastatic colorectal cancer. a phase II study of 5-day continuous venous infusion at circadian rhythm modulated rate. Eur J Cancer 29A:1280–1284
Boku N, Ohtsu A, Hyodo I et al (2007) Phase II study of oxaliplatin in Japanese patients with metastatic colorectal cancer refractory to fluoropyrimidines. Jpn J Clin Oncol 37:440–445
Kosugi C, Koda K, Ishibashi K et al (2018) Safety of mFOLFOX6/XELOX as adjuvant chemotherapy after curative resection of stage III colon cancer: phase II clinical study (The FACOS study). Int J Colorectal Dis 33:809–817
Schmoll HJ, Cunningham D, Sobrero A et al (2012) Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol 30:3588–3595
Hochster HS, Grothey A, Hart L et al (2014) Improved time to treatment failure with an intermittent oxaliplatin strategy: results of CONcePT. Ann Oncol 25:1172–1178
Yalcin S, Uslu R, Dane F et al (2013) Bevacizumab + capecitabine as maintenance therapy after initial bevacizumab + XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III “Stop and Go” study results–a Turkish Oncology Group Trial. Oncology 85:323–335
Acknowledgements
We thank Aki Komatsu (secretary at the SOAC Data Center at the Department of Frontier Surgery, Graduate School of Medicine, Chiba University) and Tomoko Nakabayashi (secretary at the Department of Surgery, Teikyo University Chiba Medical Center) for their data collection support, and all of the patients, their families, and medical staff. We thank Jane Charbonneau, DVM, and Melissa Crawford, PhD, from Edanz Group (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Chihiro Kosugi, Keiji Koda, Tadamichi Denda, and Hisahiro Matsubara: writing the protocol, data collection and interpretation, statistical analysis, and writing the paper. Keiichiro Ishibashi, Hideyuki Ishida, Kazuhiro Seike, Haruhito Sakata, Shinji Yanagisawa, Akinari Miyazaki, Wataru Takayama, Naoto Koike, and Hiroaki Shimizu: data collection and interpretation and writing paper. The first draft of the manuscript was written by Chihiro Kosugi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol and informed consent forms were approved by the ethics committee of Teikyo University, Tokyo, Japan (reference number: 12–089). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients provided written informed consent regarding the publication of their data.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kosugi, C., Koda, K., Denda, T. et al. Multicenter phase II clinical study of the efficiency and safety of capecitabine plus intermittent oxaliplatin with bevacizumab as first-line therapy in patients with metastatic colorectal cancer (VOICE trial). Int J Colorectal Dis 36, 2637–2647 (2021). https://doi.org/10.1007/s00384-021-03995-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-03995-7