Abstract
Purpose
To report planned final overall (OS) and progression-free survival (PFS) analyses from the phase II PEAK trial (NCT00819780).
Methods
Patients with previously untreated, KRAS exon 2 wild-type (WT) metastatic colorectal cancer (mCRC) were randomised to mFOLFOX6 plus panitumumab or bevacizumab. The primary endpoint was PFS; secondary endpoints included OS, objective response rate, duration of response (DoR), time to response, resection and safety. Treatment effect by tumour RAS status was a prespecified objective. Exploratory analyses included early tumour shrinkage (ETS) and depth of response (DpR).
Results
One hundred seventy patients had RAS WT and 156 had RAS WT/BRAF WT mCRC. Median PFS was longer for panitumumab versus bevacizumab in the RAS WT (12.8 vs 10.1 months; hazard ratio (HR) = 0.68 [95% confidence intervals (CI) = 0.48–0.96]; p = 0.029) and RAS WT/BRAF WT (13.1 vs 10.1 months; HR = 0.61 [95% CI = 0.42–0.88]; p = 0.0075) populations. Median OS (68% OS events) for panitumumab versus bevacizumab was 36.9 versus 28.9 months (HR = 0.76 [95% CI = 0.53–1.11]; p = 0.15) and 41.3 versus 28.9 months (HR = 0.70 [95% CI = 0.48–1.04]; p = 0.08), in the RAS WT and RAS WT/BRAF WT populations, respectively. Median DoR (11.4 vs 9.0 months; HR = 0.59 [95% CI = 0.39–0.88]; p = 0.011) and DpR (65.0 vs 46.3%; p = 0.0018) were improved in the panitumumab group. More panitumumab patients experienced ≥30% ETS at week 8 (64 vs 45%; p = 0.052); ETS was associated with improved PFS/OS. No new safety signals occurred.
Conclusions
First-line panitumumab + mFOLFOX6 increases PFS versus bevacizumab + mFOLFOX6 in patients with RAS WT mCRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First-line treatments for patients with metastatic colorectal cancer (mCRC) comprise chemotherapy combined with an epidermal growth factor receptor inhibitor (EGFRI), panitumumab or cetuximab, or the vascular endothelial growth factor inhibitor (VEGFI) bevacizumab [1]. Confirmation of wild-type (WT) tumour RAS status is essential ahead of prescribing EGFRIs [1, 2], the importance of evaluating tumour RAS status ahead of bevacizumab treatment is less clear [3, 4]. In the EU, panitumumab is indicated in combination with FOLFOX or FOLFIRI for the first-line treatment of patients with RAS WT (no mutations in exons 2, 3 and 4 of KRAS and NRAS) mCRC [5]. In the USA, panitumumab is indicated in KRAS (exon 2) WT mCRC combined with FOLFOX for first-line treatment, and as monotherapy following disease progression after prior treatment with fluoropyrimidine, oxaliplatin and irinotecan-containing chemotherapy [6]. Bevacizumab is also indicated in the EU for the treatment of patients with mCRC in combination with fluoropyrimidine-based chemotherapy [7] and in the USA with intravenous 5-fluorouracil-based chemotherapy for first- or second-line mCRC treatment and with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for second-line treatment after progression (PD) on a first-line bevacizumab-containing regimen [8].
PEAK (Panitumumab Efficacy in combination with mFOLFOX6 Against bevacizumab plus mFOLFOX6 in mCRC subjects with KRAS WT tumours) is a phase II, randomised study evaluating the efficacy and safety of first-line panitumumab + modified FOLFOX6 (mFOLFOX6) versus bevacizumab + mFOLFOX6 in patients with KRAS exon 2 WT mCRC [9]. The primary objective was to assess progression-free survival (PFS) in the KRAS exon 2 WT population; a prespecified secondary objective was to assess PFS and overall survival (OS) in patients with RAS WT mCRC. In the primary analysis of PEAK, first-line panitumumab + mFOLFOX6 was associated with numerically longer OS than bevacizumab + mFOLFOX6 in patients with RAS WT mCRC (41% OS events; median OS = 41.3 vs 28.9 months; hazard ratio (HR) = 0.63 [95% confidence intervals (CI) = 0.39–1.02]; p = 0.058) and significantly longer PFS (median PFS = 13.0 vs 9.5 months; HR = 0.65 [95% CI = 0.44–0.96]; p = 0.029) [9]. Overall response rates (ORR) by Response Evaluation Criteria In Solid Tumours (RECIST) were similar between treatments (63.6 vs 60.5%, for panitumumab vs bevacizumab groups, respectively) [9]. RECIST, however, does not fully take into account timing, depth (DpR) and duration of response (DoR), all of which may influence long-term outcomes [10, 11].
In addition to RAS mutations, other biomarkers have potential predictive and prognostic importance in mCRC. BRAF mutation status has emerged as a strong prognostic marker [12] but there is a lack of evidence to support best approaches to treatment for BRAF mutant (MT) mCRC [1, 2]. Clinical trials may, therefore, be the best option for some patients. Alternatively, a small population sub-analysis from the TRIBE trial suggested a potential OS benefit for patients with BRAF mutations receiving bevacizumab + FOLFOXIRI (n = 16) versus bevacizumab + FOLFIRI (n = 12) [13], and so, this combination may be an option for patients with good performance status.
Here, we report updated OS and PFS from a planned final analysis of PEAK data (RAS WT and RAS WT/BRAF WT populations). Exploratory analyses of tumour assessments beyond RECIST were also performed, focussing on tumour dynamics (early tumour shrinkage (ETS), depth of response (DpR) and changes in tumour load over time (RAS WT population only)).
Material and methods
Study design and patients
PEAK (NCT00819780) was a phase II, randomised (1:1), open-label first-line study in patients with previously untreated KRAS exon 2 WT mCRC. Treatment continued until PD, unacceptable toxicity, death, consent withdrawal, or investigator decision. The trial was conducted in compliance with the Declaration of Helsinki. The study protocol was approved by an independent ethics committee at each participating study centre. All patients provided informed consent before any study procedures were performed.
Key eligibility criteria for PEAK (primary analysis) included age ≥18 years; an Eastern Cooperative Oncology Group performance score of 0/1; histologically/cytologically confirmed mCRC with unresectable metastatic disease; KRAS exon 2 (codons 12 and 13) WT tumour status; one unidimensionally measurable lesion of ≥20 mm (per modified RECIST guidelines); and no prior chemotherapy, anti-EGFR therapy or bevacizumab therapy for mCRC.
Extended RAS analyses and BRAF mutation analyses
An extended RAS analysis was prespecified in PEAK [9]. In brief, banked tumour samples for patients with KRAS exon 2 WT tumours were tested for prespecified mutations in NRAS exon 2 (codons 12 and 13), and KRAS and NRAS exons 3 (codons 59 and 61) and 4 (codons 117 and 146). Mutations were also assessed in exon 15 (codon 600) of BRAF (exploratory analysis). Mutation status was assessed using bidirectional Sanger sequencing and WAVE-based SURVEYOR® scan kits (Transgenomic; Omaha, NE, USA), as reported previously [12].
Efficacy assessments
Endpoints reported here include PFS, OS, ORR, DoR, time to response (TTR), resection rates, DpR, change in tumour load versus baseline (over time), and ETS. Tumour response was assessed by the investigator every 8 weeks (±7 days) using modified RECIST (version 1.0; there was no independent review). ETS was defined as the proportion of patients with tumour shrinkage at week 8; two cutoff values were used, ≥30 and ≥20%. Tumour shrinkage was defined as the change in sum of the longest diameters (SLD) of all target lesions. DpR was defined for each patient as the percentage of tumour shrinkage at nadir or progression. DpR has a positive value for tumour reduction, a negative value for tumour growth and is zero for no change. If shrinkage occurred during treatment, then DpR was the greatest percentage tumour shrinkage observed at nadir versus baseline. In all other cases, DpR was defined as the percentage of tumour change at progression. If progression did not occur, DpR was not defined as PFS could not be defined. Changes in tumour load were calculated as mean (95% CI) percentage change from baseline in the SLD of all target lesions over time.
Safety assessments
Patients were followed-up for safety until 30 days after the last dose of study drug. Adverse events (AEs) were graded using Common Terminology Criteria for Adverse Events (version 3.0), with modifications for specific skin- and nail-related toxicities.
Statistical analyses
The primary endpoint in PEAK was PFS; secondary endpoints included OS, ORR, DoR, TTR, resection rates, and safety (all reported here for the RAS WT population). Six-month PFS and 2-year OS rates by resection status were calculated by treatment (exploratory analysis). Exploratory analyses of DpR, changes in tumour load versus baseline and ETS, were performed for the RAS WT population; median PFS and OS were calculated for patients with/without ≥30 and ≥20% ETS, by treatment and overall. Top-line efficacy results (PFS, OS and ORR) were also reported for the RAS WT/BRAF WT and RAS WT/BRAF MT populations.
The treatment effect HR for panitumumab relative to bevacizumab combined with mFOLFOX6 and the associated 95% CIs were estimated from a Cox model stratified by previous adjuvant oxaliplatin therapy. Kaplan-Meier curves were generated for all time-to-event endpoints.
The primary analysis of PEAK was performed in 2012 when the target of ~168 PFS events was achieved [9]. This PFS event goal was expected to be achieved with recruitment of approximately 280 patients. The current final analysis was planned to occur 36 months after the last patient enrolled. No formal hypothesis testing was planned; all reported p values are descriptive.
The prespecified extended RAS analysis included all intent-to-treat (ITT) patients with RAS data available. The extended RAS statistical analysis plan was developed before the data analysis was conducted and no biomarker testing results were available to the study team before plan approval.
Results
Patients
Between April 2009 and December 2011, 285 patients with KRAS exon 2 WT mCRC were enrolled from 60 sites; 142 patients were randomly assigned to panitumumab + mFOLFOX6 and 143 to bevacizumab + mFOLFOX6 (primary analysis ITT set [Fig. 1]). Overall, 278 patients received treatment. Samples from 250 patients in the ITT population (88%) underwent extended RAS analysis, with 233 (82%) having a result. Twelve patients were identified as having KRAS exon 2 mutations upon additional testing, giving a KRAS exon 2 WT population of 221. Among these 221 patients, 170 (77%) had RAS WT mCRC. BRAF mutations were found in tumours from 14 patients (panitumumab + mFOLFOX6 n = 11; bevacizumab + mFOLFOX6 n = 3), all of whom had RAS WT mCRC.
Baseline demographics and disease characteristics were generally similar between treatments in both the RAS WT and RAS WT/BRAF WT populations (Table 1). The mean (standard deviation) actual follow-up time was 134.1 (73.8) weeks in the panitumumab arm and 115.7 (71.0) weeks in the bevacizumab arm. Overall, the median duration of panitumumab treatment was 14.0 cycles in both the RAS WT and RAS WT/BRAF WT populations. The median duration of bevacizumab treatment was 11.0 and 11.5 cycles in the RAS WT and RAS WT/BRAF WT populations, respectively.
Efficacy
At the time of analysis, 73 versus 85% of patients with RAS WT mCRC receiving panitumumab + mFOLFOX6 versus bevacizumab + mFOLFOX6, respectively, had PFS events (Table 2). In the RAS WT population, PFS was longer in the panitumumab versus bevacizumab group (median = 12.8 vs 10.1 months; HR = 0.68 [95% CI = 0.48–0.96]; p = 0.0292) (Fig. 2a).
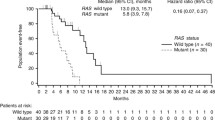
Mortality rates in the RAS WT population at the time of analysis were 65 versus 71% for the panitumumab + mFOLFOX6 versus bevacizumab + mFOLFOX6 groups, respectively (Table 2). In the RAS WT population, median OS was 36.9 versus 28.9 months in the panitumumab versus bevacizumab groups, respectively (HR = 0.76 (95% CI = 0.53–1.11)); p = 0.15) (Fig. 2b). Although some patient subpopulations were small, the trend (not statistically significant) towards improved OS in the panitumumab group was generally observed across subpopulations defined by baseline covariates (Online Resource Fig. A1). Similar proportions of patients in the panitumumab and bevacizumab groups subsequently crossed over to anti-VEGF and anti-EGFR therapies, respectively (Table 2).
Overall, 169 patients with RAS WT mCRC were included in the ORR analysis; ORRs were similar in the panitumumab + mFOLFOX6 and bevacizumab + mFOLFOX6 arms (65 vs 60%, respectively; odds ratio = 1.12 (95% CI = 0.56–2.22); p = 0.86) (Table 2).
Similar observations for PFS (median = 13.1 vs 10.1 months; HR = 0.61 (95% CI = 0.42–0.88); p = 0.0075), OS (median = 41.3 vs 28.9 months; HR = 0.70 (95% CI = 0.48–1.04); p = 0.08) and ORR (65 vs 62%) were found for the panitumumab versus bevacizumab groups, respectively, in the RAS WT/BRAF WT population (Table 2; Online Resource Fig. A2). The efficacy of panitumumab and bevacizumab in patients with RAS WT/BRAF MT disease could not be determined due to the small number of patients in this subpopulation (n = 11 and n = 3, respectively; Online Resource Table A1).
In patients with RAS WT mCRC, median TTR was numerically shorter, median DoR longer and median DpR greater in the panitumumab + mFOLFOX6 versus bevacizumab + mFOLFOX6 group (Table 3). Resection rates and outcomes following resection were similar between treatments for the RAS WT population, although sample sizes were small for this analysis (Table 3).
The mean percentage change from baseline in tumour load in the RAS WT population appeared to favour panitumumab at all measured time points, although the CIs were wide (Fig. 3). Figure 3 shows tumour data at each scheduled visit; following PD, patients were only followed up for survival and no further CT scans were analysed.
Overall, 154 patients with RAS WT mCRC had tumour shrinkage data available at baseline and week 8. Compared with the bevacizumab + mFOLFOX6 arm, more patients in the panitumumab + mFOLFOX6 group experienced ≥30% ETS (Table 4). Numerically, more patients in this group also had ≥20% ETS. For those achieving ≥30 or ≥20% ETS, numerically longer PFS and OS were seen in the panitumumab versus bevacizumab arm (Table 4; Online Resource Fig. A3). ETS (≥30 or ≥20%) was associated with numerically longer PFS and OS, irrespective of treatment received (Online Resource Table A2). When treatment arms were combined, achievement of ≥30 versus <30% ETS was associated with longer PFS (median = 12.9 vs 9.8 months; HR = 0.60 (95% CI = 0.42–0.87); p = 0.0065) and OS (median = 43.4 vs 24.3 months; HR = 0.44 (95% CI = 0.30–0.65); p < 0.0001). Similar results were seen when combined data were analysed using the ≥20% ETS cutoff (median PFS = 13.0 vs 9.5 months; HR = 0.55 [95% CI = 0.37–0.81]; p = 0.0029; median OS = 39.1 vs 21.2 months; HR = 0.39 [95% CI = 0.26–0.59]; p < 0.0001).
Safety
In the RAS WT safety analysis population, similar proportions of patients in the panitumumab and bevacizumab groups experienced AEs (100 vs 100%), worst grade 3 or 4 AEs (90 vs 73%), serious AEs (43 vs 40%), fatal AEs (5 vs 9%) and discontinuations (any drug) due to AEs (29 vs 30%) (Online Resource Table A3). Overall, 20% of patients discontinued panitumumab and 25% discontinued bevacizumab treatment due to an AE. In the panitumumab arm, four fatal AEs (5%) were reported: cardiac arrest, pneumonia aspiration, respiratory failure and sepsis (n = 1 for each). In the bevacizumab arm, seven fatal AEs (9%) were reported: cardiac arrest, sepsis, cardio-respiratory arrest, intestinal obstruction, intestinal perforation, septic shock and small intestinal perforation (n = 1 for each). Overall, 36 versus 3% of patients in the panitumumab versus bevacizumab groups had grade ≥3 skin/subcutaneous tissue disorders; 5 versus 18% of patients in these groups, respectively, had grade ≥3 vascular disorders. There was a ≥5% difference in incidence rate between treatment groups for the following grade ≥3 AEs (panitumumab vs bevacizumab): rash (15 vs 0%), hypomagnesaemia (8 vs 0%), stomatitis (7 vs 0%), decreased appetite (6 vs 1%), dehydration (6 vs 1%), acne (5 vs 0%), dermatitis acneiform (5 vs 0%), deep vein thrombosis (2 vs 8%), and hypertension (0 vs 8%) (Online Resource Table A3).
Discussion
The efficacy results from this final analysis of PEAK were consistent with the results of the primary analysis of this trial [9]. Patients with RAS WT tumours receiving panitumumab + mFOLFOX6 had longer PFS (12.8 vs 10.1 months, p = 0.029) and DoR (11.4 vs 9.0 months, p = 0.011) compared with those receiving bevacizumab + mFOLFOX6. ORR (65 vs 60%, p = 0.86) and resection rates (14 vs 11%) were similar between treatments. Exploratory analyses of DpR (median 65 vs 46%, p = 0.0018) and ETS (≥30% ETS 64 vs 45%, p = 0.052) suggested improvements in these measures for the panitumumab versus bevacizumab group. In line with previous reports [10, 11, 14, 15], ETS appeared to be associated with improved PFS and OS, irrespective of first-line treatment received. A limitation to this study is that radiologic assessment was performed by the investigator and no independent radiologic review was performed.
In the RAS WT population, median OS was 8 months longer in the panitumumab group (36.9 vs 28.9 months; HR = 0.76 [95% CI = 0.53–1.11]; p = 0.15). Median OS was further extended in patients with RAS WT/BRAF WT tumours receiving panitumumab (41.3 months), but remained the same as for the RAS WT population in RAS WT/BRAF WT patients receiving bevacizumab (28.9 months). This is of note because patients with BRAF MT mCRC have a poor prognosis [12] and there is a small imbalance in the number of patients with BRAF MT tumours in this study (panitumumab arm n = 11; bevacizumab arm n = 3). However, the primary endpoint in PEAK was not OS and this trial was not powered to detect an OS increase, which may explain why the observed difference between arms was not statistically significant. The overall findings from PEAK nonetheless suggest an OS benefit for EGFRIs + chemotherapy versus bevacizumab + chemotherapy, which is consistent with results from FIRE-3 [16] and a congress presentation of limited data for the FOLFOX arm of the CALGB/SWOG 80405 study (full publication not yet available) [17]. This is also in line with results from two recent meta-analyses of these trials, which included primary analysis data from PEAK. Both meta-analyses demonstrated significantly improved ORR and OS with first-line anti-EGFR compared with anti-VEGF therapy in patients with RAS WT mCRC [18, 19]. The HR (95% CI) for OS was 0.77 (0.63–0.95) in favour of the EGFRI in both analyses. Similar results were also observed in a meta-analysis including seven first-line trials comparing panitumumab or cetuximab plus chemotherapy with chemotherapy alone or chemotherapy plus bevacizumab [20].
The median OS of 41.3 months achieved in patients with RAS WT/BRAF WT mCRC receiving panitumumab in PEAK, compares well with other data for first-line biologics combined with doublet chemotherapy [12, 13]. Data have recently been reported for triplet chemotherapy combined with biologics in several studies including TRIBE [13, 21] (bevacizumab), TRIP [22] and VOLFI [23] (panitumumab) and MACBETH [24] (cetuximab), indicating that FOLFOXIRI may offer superior efficacy over doublet chemotherapy in the first-line setting. However, improved outcomes are achieved at the expense of increased grade 3/4 toxicity, including neutropenia, diarrhoea, stomatitis and neurotoxicity, which may limit the use of such regimens in clinical practice.
Although the OS results from PEAK are now more mature (68 and 66% deaths in the RAS WT and RAS WT/BRAF WT populations, respectively), these results need to be considered in context of the fact that PEAK is a phase II trial. There are several possible reasons for the prolonged OS observed. For example, the improved DoR in the panitumumab + mFOLFOX6 arm could be linked with the improved PFS and numerically improved OS seen in this arm. Likewise, the improved DpR, ETS and tumour load changes in the panitumumab versus bevacizumab arm could also play a role in improving long-term outcomes, as has been suggested in other reports [11, 25]. It is unlikely that the numeric, between-treatment OS differences were driven by resection, as resection rates in patients with RAS WT tumours were balanced between groups. It is also unlikely to be driven by differences in tumour side as the proportion of patients with left- and right-sided disease was well-balanced between treatment groups. As was suggested in FIRE-3 [26], the impact of treatment sequence could also be important, given that the proportions of patients switching biologic approach were balanced between arms (i.e. EGFRI to VEGFI or VEGFI to EGFRI). Preclinical data suggest that first-line EGFRI treatment may sensitise tumours to subsequent therapy, whereas first-line VEGFI may desensitise tumours [27,28,29]. This provides a biological rationale for first-line EGFRI therapy followed by second-line VEGFI therapy.
As well as comparative efficacy, safety/tolerability and cost-effectiveness of the available treatments need to be considered when choosing first-line therapy for patients with RAS WT mCRC. Updated data for the RAS WT population in PEAK suggest that the safety profiles of the two treatments are consistent with previously reported studies and no new toxicities or safety signals were identified. The main tolerability differences were due to known class effects of these treatments, e.g. skin rash/toxicities and hypomagnesaemia with the EGFRI and deep vein thrombosis and hypertension with bevacizumab. Discontinuation rates due to AEs were similar between arms (29 vs 30%, respectively).
Tumour biomarker status is increasingly being used to guide treatment decisions in mCRC and is a rapidly developing area of research. By targeting drugs only to those patients most likely to benefit, predictive biomarkers may improve clinical outcomes and also potentially reduce overall treatment costs. The availability of banked tumour samples from clinical studies is important to permit prospective, prospective-retrospective or retrospective analyses to be performed to test new biomarker hypotheses [30]. Analysis of data from the phase III PRIME study of panitumumab + FOLFOX4 confirmed the importance of RAS testing beyond KRAS in patients with mCRC and was possible due to the availability of RAS data for 90% of the KRAS WT tumour samples [12]. The RAS ascertainment rate in the PEAK study was also high (82%) in comparison to currently available data from other first-line mCRC studies comparing EGFRIs versus VEGFIs (FIRE-3 = 69% [16]; CALGB 80405 = 55% [17]), highlighting the importance of including the availability of paraffin-embedded tumour tissue for biomarker testing as a requirement for study entry. Mandating baseline biopsy and planning the conduct of prospective/prespecified biomarker analyses in clinical trials, can improve the robustness of the study data and future analyses. While tumour RAS status is undoubtedly a valuable predictive marker for response to EGFRIs, it remains important to investigate the roles of other potential biomarkers that could improve patient selection (e.g. epiregulin/amphiregulin, PIK3CA, PTEN). Data from the PRIME study indicated that BRAF mutations are linked with poor outcomes irrespective of treatment received [12]. RAS mutations do not appear to be prognostic for patients with mCRC.
In summary, ORR by RECIST is the accepted standard for assessing response in solid tumours, but has limitations in terms of assessing dynamic tumour burden changes. Although ORRs were similar between treatments in PEAK, tumour responses in the panitumumab arm appeared to occur earlier, last longer and be deeper compared with those occurring in the bevacizumab arm in these exploratory analyses. More patients receiving panitumumab + mFOLFOX6 versus bevacizumab + mFOLFOX6 had ≥30% ETS; ETS of ≥30 or ≥20% appeared to be associated with improved PFS and OS, irrespective of treatment received. PFS was also significantly improved for patients with RAS WT mCRC receiving panitumumab + mFOLFOX6 versus bevacizumab + mFOLFOX6. For panitumumab versus bevacizumab, median OS was 8.0 months longer in the RAS WT (36.9 vs 28.9 months) and 12.4 months longer in the RAS WT/BRAF WT (41.3 vs 28.9 months) populations, respectively (neither difference being statistically significant). Based on these data, panitumumab + mFOLFOX6 can be considered an effective first-line treatment for patients with RAS WT mCRC. A phase III trial is warranted to more fully evaluate any potential differences between panitumumab and bevacizumab treatment in the first-line setting.
References
National Comprehensive Cancer Network® (2015) Clinical practice guidelines in oncology (NCCN Guidelines®): colon cancer version 1.2015. Available at: www.nccn.org/. Access date 15 March 2017
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D (2014) Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii1–iii9
Koike J, Ushigome M, Funahashi K, Shiokawa H, Kaneko T, Arai K, Matsuda S, Kagami S, Suzuki T, Kurihara A, Shimada H, Kaneko H (2014) Significance of KRAS mutation in patients receiving mFOLFOX6 with or without bevacizumab for metastatic colorectal cancer. Hepato-Gastroenterology 61:2222–2226
Price TJ, Bruhn MA, Lee CK, Hardingham JE, Townsend AR, Mann KP, Simes J, Weickhardt A, Wrin JW, Wilson K, Gebski V, Van HG, Robinson B, Cunningham D, Tebbutt NC (2015) Correlation of extended RAS and PIK3CA gene mutation status with outcomes from the phase III AGITG MAX STUDY involving capecitabine alone or in combination with bevacizumab plus or minus mitomycin C in advanced colorectal cancer. Br J Cancer 112:963–970
European Medicines Agency (2015) Vectibix® (panitumumab) Summary of Product Characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000741/WC500047710.pdf. Access date 15 March 2017
Amgen Inc. (2015) Vectibix® prescribing information. Available at: http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf. Access date 15 March 2017
European Medicines Agency (2015) Avastin® (bevacizumab) summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf. Access date 15 March 2017
Genentech Inc. (2015) Avastin® (bevacizumab) prescribing information; South San Francisco, CA: Genentech, Inc. Available at: http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf. Access date 15 March 2017
Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY (2014) PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 32:2240–2247
Heinemann V, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Giessen C, Möhler M, Stintzing S (2014) Independent radiological evaluation of objective response, early tumour shrinkage, and depth of response in FIRE-3 (AIO KRK-0306). Ann Oncol 25(Suppl 4):ii117 (Abstract O-0030)
Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, Cortesi E, Tomasello G, Spadi R, Zaniboni A, Tonini G, Barone C, Vitello S, Longarini R, Bonetti A, D'Amico M, Di DS, Granetto C, Boni L, Falcone A (2015) Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol 26:1188–1194
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023–1034
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Carlomagno C, Allegrini G, Chiara S, D'Amico M, Granetto C, Cazzaniga M, Boni L, Fontanini G, Falcone A (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16:1306–1315
Piessevaux H, Buyse M, Schlichting M, Van CE, Bokemeyer C, Heeger S, Tejpar S (2013) Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 31:3764–3775
Douillard JY, Siena S, Peeters M, Koukakis R, Terwey JH, Tabernero J (2015) Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer 51:1231–1242
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Muller S, Link H, Niederle N, Rost A, Hoffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075
Lenz H, Niedzwiecki D, Innocenti F, Blanke C, Mahoney MR, O'Neil BH, Shaw JE, Polite B, Hochster H, Atkins J, Goldberg R, Mayer R, Schilsky R, Bertagnolli M, Venook A (2014) CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with expanded ras analyses untreated metastatic adenocarcinoma of the colon or rectum (mCRC). Ann Oncol 25(Suppl 4):ii117 (Abstract 501O)
Khattak MA, Martin H, Davidson A, Phillips M (2015) Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer 14:81–90
Heinemann V, Rivera F, O'Neil BH, Stintzing S, Koukakis R, Terwey JH, Douillard JY (2016) A study level meta-analysis of efficacy data from head-to-head first-line trials of epidermal growth factor receptor inhibitors vs bevacizumab in patients with RAS wild-type metastatic colorectal cancer. Eur J Cancer 67:11–20
Pietrantonio F, Cremolini C, Petrelli F, Di Bartolomeo M, Loupakis F, Maggi C, Antoniotti C, de Braud F, Falcone A, Iacovelli R (2015) First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 96:156–166
Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371:1609–1618
Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, Cremolini C, Schirripa M, Vivaldi C, Aprile G, Zaniboni A, Bracarda S, Fontanini G, Sensi E, Lupi C, Morvillo M, Zagonel V, Falcone A (2013) FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 24:2062–2067
Martens U, Wessendorf S, Riera Knorrenschild J, Buechner-Steudel P, Florschuetz A, Atzpodien J, Keller R, Greeve J, Kanzler S, Ettrich T, Lindig U, Egger M, Hebart H, Geissler M (2015) AIO-KRK-0109: a randomized phase II trial of panitumumab plus FOLFOXIRI or FOLFOXIRI alone as 1st-line treatment in RAS-wild-type metastatic colorectal cancer (mCRC). Ann Oncol 6(Suppl 4) Abstract 2049
Cremolini C, Loupakis F, Salvatore L, Lonardi S, Battaglin F, Gamucci T, Grande R, Di Fabio F, Rojas Llimpe FL, Ferrari L, Casagrande M, Lucchesi S, Tonini G, Banzi M, Valsuani C, Corsi DC, Fontanini G, Tomcikova D, Falcone A (2014) Modified FOLFOXIRI plus cetuximab (cet) as induction treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): preliminary results of the phase II randomized Macbeth trial by GONO group. J Clin Oncol 32(Suppl 5S) Abstract 3596
Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR (2015) Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 51:1927–1936
Modest DP, Stintzing S, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Mohler M, Jung A, Kirchner T, Heinemann V (2015) Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol 33:3718–3726
Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS (2001) Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res 61:5090–5101
Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, Melisi D, De Vita F, De Placido S, Bianco AR, Tortora G (2004) Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res 10:784–793
Wainberg ZA, Drakaki A (2015) The importance of optimal drug sequencing in metastatic colorectal cancer: biological rationales for the observed survival benefit conferred by first-line treatment with EGFR inhibitors. Expert Opin Biol Ther 15:1205–1220
Peeters M, Karthaus M, Rivera F, Terwey JH, Douillard JY (2015) Panitumumab in metastatic colorectal cancer: the importance of tumour RAS status. Drugs 75:731–748
Acknowledgements
The authors wish to acknowledge all of the patients who participated in the PEAK (20070509) study, the PEAK study investigators and their staff for their participation in the conduct and reporting of this study, the study and biostatistics team at Amgen, and the medical writing support provided by Dawn Batty PhD of Bioscript Medical Ltd. (funded by Amgen [Europe] GmbH). Amgen Inc. sponsored the PEAK study and was involved in design, conduct, data collection, analysis and data interpretation.
Funding
Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
FR has acted on advisory boards and received research funding from Amgen, during the course of this study. He has also acted on advisory boards or received research funding from Bayer, Lilly, Merck-Serono, Merck Sharp and Dohme, Roche, Sanofi, and Servier, outside of the submitted work. MK reports personal fees from Amgen and Roche, outside the submitted work. JRH reports personal fees from Amgen and Roche, outside the submitted work. IS has acted on advisory boards/received honoraria from Amgen, Roche, Merck-Serono, Sanofi, Bayer, Ipsen and Novartis. FF has acted on advisory boards/received honoraria from Novartis and Nutricia, and received research grants from Amgen, outside the submitted work. GF reports grants and personal fees from Amgen, during the course of the study. JLC reports research grants from Amgen, outside the submitted work. XG is an employee of Amgen Inc. GD is an employee of Amgen (Europe) GmbH. LSS has acted as a consultant and on a speaker’s bureau for Amgen, outside the submitted work.
Statement of human rights
PEAK (NCT00819780) was conducted in compliance with the Declaration of Helsinki. The study protocol was approved by an independent ethics committee at each participating study centre.
Informed consent
All patients provided informed consent before any study procedures were performed.
Electronic supplementary material
ESM 1
(DOCX 1211 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rivera, F., Karthaus, M., Hecht, J.R. et al. Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis 32, 1179–1190 (2017). https://doi.org/10.1007/s00384-017-2800-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-017-2800-1