Abstract
Purpose
The main operative method in familial adenomatous polyposis (FAP) patients is restorative proctocolectomy with “J”-shaped pouch and temporary loop ileostomy. The aim of the study was the analysis of the frequency of the dysplasia and inflammation in the intestinal pouch and prognosis of the clinical course in FAP patients after restorative proctocolectomy.
Methods
A group of 165 FAP patients (86 females and 79 males, mean age 22.49 ± 12) subjected to a restorative proctocolectomy in the years 1985–2009 was analyzed. Clinical data coming from follow-up observation in the period of 2004–2009 were evaluated. In all patients, clinical examination and endoscopy with polypectomy and/or biopsy of pouch mucosa were done.
Results
The mean time of pouchitis occurrence after an ileal pouch-anal anastomosis was 6 months. Mean time for low-grade dysplasia was 14 months. The time difference of low-grade dysplasia after the above procedure as compared to pouchitis alone was substantial. Mean time for high-grade dysplasia was 16 months and for neoplasia even 19 months. It was estimated that early pouchitis happening within the first year after surgery occurs in 5% of patients, low-grade dysplasia 4 years later in 7% of cases, high-grade dysplasia 7 years later in around 10% of patients and neoplasia 14 years after surgery in 15% of cases.
Conclusions
In conclusion, the Polyposis Registry encompassing whole country is the best way of controlling FAP patients. The regular lifelong endoscopic monitoring gives the opportunity of the early detection of the dysplasia and can protect against neoplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial adenomatous polyposis (FAP) is a rare genetic disorder characterized by multiple colorectal polyps undergoing malignant transformation. FAP incidence in European Union countries estimated by European Medicines Agency in 2009 is three to ten new cases per 100,000 that means 11,300–37,600 FAP cases EU-wide [1]. Because of the genetic background of the disease, there is no causative treatment. Surgical treatment is devoted to avoid further neoplasia. The main operative method in FAP patients is restorative proctocolectomy with “J”-shaped pouch and temporary loop ileostomy. This is a very extensive and radical procedure [2], not yet fully protecting against malignant transformation in the rectal remnant and anastomosis [3]. Another widely accepted surgical procedure is colectomy with ileo-anal anastomosis performed when there are few polyps in the rectum. Some studies suggest that there should not be more than ten rectal polyps [4], others that no more than five [5]. A great advantage of this method is the preservation of the rectal innervations, subsequent better quality of life [6] and fewer problems with erection and ejaculation.
In closely selected cases, especially with fewer polyps than in FAP, namely in attenuated familial adenomatous polyposis and also in patients disagreeing to surgery, regular endoscopic polypectomies are necessary. This is not a standard procedure and is not recommended as the treatment of choice in polyposes [7].
Another significant issue in patients after an ileal pouch-anal anastomosis or ileorectal anastomosis is a recurrence of adenomas and malignancy in the rectal remnant, anastomosis or pouch. Frequency and dynamics of pouch dysplasia is being still discussed. There is a high discrepancy between the studies reporting the incidence of dysplasia in 8–74% of patients undergoing proctocolectomy due to FAP [5, 8].
Patients and methods
A group of 165 FAP patients subjected to restorative proctocolectomy in the years 1985–2009 was retrospectively analysed. A group of 86 females and 79 males with the mean age of 22.49 (±12) was carefully selected from the total number of admitted patients to the Department of General, Gastroenterological and Endocrynological Surgery and Department of General and Colorectal Surgery, Poznan University of Medical Sciences, Poland. Follow-up investigations were done in both departments and, in some cases, in four other clinical centers, easy accessible for the patients. Clinical data coming from follow-up visits in the years 2004–2009 as well as medical documentation from these centers were also evaluated.
Operative technique
The first group of 13 patients was collected to the proctocolectomy with “J”-pouch with manual ileorectal anastomosis and mucosectomy. The remaining 152 patients underwent the same but stapled procedures: 3 of them had restorative proctocolectomy with “J”-pouch without temporary ileostomy, 142 had two-stage procedure with ileostomy and 7 had three-stage surgery (colectomy with end-ileostomy, “J”-pouch construction with temporary ileostomy and closure of ileostomy). Another 13 patients demonstrated acute surgery indications, such as mechanical bowel obstruction and bleeding and were operated immediately: nine of them underwent three-stage procedures and four had two-stage surgery.
Endoscopic examination
It is a standard since the onset of restorative proctocolectomy performed at our department that we follow-up our patients at 1-year intervals performing both endoscopic and histologic evaluation of the pouch as well as gastroscopy, abdominal and thyroid ultrasound and abdominal CT to exclude possible extracolonic manifestations of FAP. In the majority of cases, endoscopy was performed in the out-patient clinic with a rigid sigmoidoscope measuring either 8 or 15 mm in diameter. The choice of its size depended on the pouch-anal anastomosis diameter diagnosed by digital per rectum examination. In some cases, a flexible colonoscope was used. In particular instances, in patients with severe pouchitis, anastomotic stricture and inflammation, endoscopic evaluation took place under general anaesthesia. Endoscopy was performed by four experienced surgeons. Each time new polyps were subjected to polypectomy and histologic examination. Small lesions of the mucosa such as minute polyps and fold thickenings were destroyed by electrocoagulation.
Specimens were taken from the mucosa as the standard procedure to assess its transformation and verify inflammation based on pouchitis disease activity index (PDAI). Usually, two biopsies were sampled from the pouch wall and its midportion also macroscopic lesions of the pouch, ulcerations, fold thickening, inflammation, etc. Due to a lack of routinely taken specimens and the absence of standardized clinical protocols, this report does not contain an analysis of changes within the rectal canal (cuffitis, dysplasia or neoplasia). The specimens from anal transitional zone were taken only in case of the suspected macroscopical lesions as the polyps or ulcers, totally from 85 patients.
The protocol was fully accepted by all patients and did not affect their life quality. None of the patients refused endoscopy and biopsy sampling.
Pouchitis, dysplasia and neoplasia diagnosis
The pouchitis was recognized with scores at least 7 used the 18-point PDAI [9]. Chronic pouchitis was diagnosed in patients with at least three episodes of pouchitis within 12 months with at least one incident confirmed endoscopically and histologically. Diagnosis of the next episodes of pouchitis was based on both endoscopic and histologic evaluation or solely endoscopic one, stool granulocytic enzyme activity and clinical symptoms. Histologic assessment was performed in all cases by two independent fellow pathologists. In all cases, the pathologists were blinded to the clinical details such as the time from pouch construction to biopsy, clinical symptoms and the macroscopic results of the endoscopy. Inflammation severity was classified according to Moskowitz classification and villous atrophy according to Laumonier scale. Either low- or high-grade dysplasia and neoplasia were diagnosed by the same fellow pathologists. In this study, patients were divided into groups with low-grade dysplasia, high-grade dysplasia and or neoplasia according to the severity of the above-mentioned lesion, for instance a patient with both neoplasia and foci of dysplasia was qualified for the neoplasia group.
Pouch failure analysis
Necessity to excise the ileal pouch or exteriorize loop ileostomy was considered to be the pouch failure. The above procedures were performed in our department in seven cases and we found one such operation carried out outside our centre as confirmed by medical documentation.
Statistical analysis
The frequency of dysplasia, neoplasia and pouch failure occurring at different times after surgery is presented in tables. Univariate statistical analysis of frequency of certain conditions after surgical procedure was compared by Fisher’s exact test. Multivariate analysis was performed with logistic regression. Relationship between pouchitis and high-grade and low-grade dysplasia was evaluated. The likelihood ratio of dysplasia as an unfavourable factor in patients with pouchitis was estimated by Kaplan–Meier’s method. Kaplan–Meier’s curves illustrate when to expect early complications in a group of patients and when they occur in 50% of cases. Statistical analyses were performed with the aid of StatXact (Cytel Inc) and MedCalc Software.
Results
In the years 2004 and 2009, a number of 496 endoscopic examinations was performed in our department and in 27 cases in four other centers. All of them took place from 2 to 19 years after restorative proctocolectomy. There were no serious complications after endoscopy except three cases of discrete bleeding after obtaining biopsy (resolved spontaneously in two patients, one managed by electrocoagulation). The majority of polypectomies—80%— were performed in our out-patient clinic; in 16% of cases, patients underwent 1-day hospital admission. In 4% of cases, patients stayed at hospital for more than 1 day. There were no complications during follow-up visits reported by outside hospitals. The summary of the frequency of the pouchitis, low-grade dysplasia, high-grade dysplasia, pouch neoplasia, pouch excision and re-stoma in FAP patients after restorative proctocolectomy depend on the time after surgery is showed in Table 1.
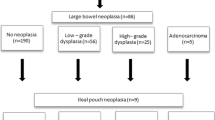
Low-grade dysplasia was recognized in 21 specimens taken from 13 patients, high-grade dysplasia in 11 specimens taken from eight patients. The neoplasia has occurred in six specimens taken from five patients. The dysplastic lesions occurred totally in 26 patients. In this group, 17 patients presented some extraintestinal manifestation: gastric and duodenal polyps (12 patients), desmoids (five patients), osteomas (four patients) and thyroid malignancy (one patient). In some patients, more than one extraintestinal manifestation occurred. In specimens taken from anal transitional zone in 85 patients, there were 19 cases of low-grade dysplasia, ten cases of the high-grade dysplasia and one case of cancer.
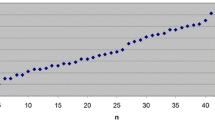
The odds ratio for low-grade dysplasia in a group with pouchitis was 1.93 and that of high-grade dysplasia was 2.61. The odds ratio for neoplasia was 1.09. Logistic regression did not confirm any statistically significant correlation between pouchitis, low- or high-grade dysplasia and neoplasia. The mean time of pouchitis occurrence after an ileal pouch-anal anastomosis was 6 months. Mean time for low-grade dysplasia was estimated to 14 months. The time difference of low-grade dysplasia after the mentioned procedure as compared to pouchitis alone was substantial. Mean time for high-grade dysplasia was 16 months and for neoplasia even 19 months. That might be the main reason, why there is no statistical significance between their mean times of occurrence and that of low-grade dysplasia (Fig. 1).
Kaplan–Meier’s curves of complications after surgery (Fig. 1) present cumulative risk of them. According to this study, pouchitis may happen in 30% of patients in a mean time of 4 years after an ileal pouch-anal anastomosis. The estimated frequency of low-grade dysplasia 4 years after surgery is 4% and 50% 15 years after it (Fig. 2). The same percentage of patients but with high-grade dysplasia develops it 2.5 years later, that is to say 17.5 years after IPAA and with neoplasia 18.5 years after the above procedure.
It was estimated that early pouchitis happening within the first year after surgery occurs in 5% of patients’ group, low-grade dysplasia 4 years later in 7% of cases, high-grade dysplasia 7 years later in around 10% of patients and neoplasia 14 years after surgery in 15% of cases.
Comparing the Kaplan–Meier’s curves showed no significant differences between the group with pouchitis and without pouchitis, as well as with dysplasia and neoplasia were found. Statistically significant differences between groups with pouchitis and low-/high-grade dysplasia and neoplasia were found (p < 0.05).
Discussion
The majority of FAP patients underwent restorative proctocolectomy with ileal pouch-anal anastomosis. This is a relatively safe procedure with an acceptable incidence of complications, good functional results securing good quality of life [5]. According to Utsunomiya, one of the proponents of this method, it had to safeguard a patient against neoplasia risk in the large bowel. Nowadays, it is clear, a risk of adenocarcinoma in the anal canal (10–31%) and ileal pouch (8–62%) is still present. That is why lifelong endoscopic monitoring is required [5, 10]. There is still a problem of evaluation of dysplasia risk, its occurrence time after surgery and individualization of prognosis. There is a discrepancy of data in recent bibliography. On one side, there are reports of high incidence of dysplasia in FAP patients after restorative proctocolectomy. Moussata et al. found foci of dysplasia in 74% of cases 5 years after surgery [8], Tajik et al. in 66% [11]. In a study on a large group of 254 patients, Friedrich et al. estimated a cumulative risk of adenoma at 45% 10 years after surgery and for adenocarcinoma at 1.9% [12]. Parc et al. in a group of 85 patients estimated the risk of adenoma formation at 35% and did not notice malignant transformation. Adenoma development was assessed 5, 10 and 15 years after surgery adequately at 7%, 35% and 75% (Fig. 3) [13]. On the other side, there are also astonishing results of some studies on FAP patients after restorative proctolocectomy in which dysplasia was found very rarely in about 8–10% of cases [5, 14]. It should be remembered that in 5% of FAP patients subjected to restorative proctocolectomy, the risk of ileal pouch adenocarcinoma, usually in the distal portion of the pouch, exists independently of the surgical procedure [15].
In this study, dysplasia and neoplasia were found in 15.7% of cases. Their distribution varied at different times after surgery. In a group 0–10 years after the surgery, low-grade and high-grade dysplasias were present in 3.6% of patients, but in a group 10 years after operation in 28% of cases.
A few factors such as time after surgery and rigid or flexible endoscopy employment caused some discrepancy in bibliography as to the frequency of dysplasia. Centers which perform chromoendoscopy have a higher frequency of dysplasia finding [8, 12]. Another important factor reducing the percentage of high-grade dysplasia and neoplasia is the frequency of follow-up visits and electrocoagulation of small superficial lesions without histological evaluation. There is also a discrepancy in findings in follow-up visits in FAP patients after proctocolectomy. Some authors analyze the incidence of dysplasia and neoplasia, some other authors the incidence of polyps. It should be stressed that we cannot associate polyps strictly with foci of dysplasia and that dysplasia does not always manifests itself in the form of a polyp. Anal transitional zone inclusion into the at risk zones for dysplasia also makes its incidence relatively higher. This mucosal remnant has histologic manifestations of the rectal mucosa [16] and after a stapled operative technique, it usually measures 0.5–2 cm. Even mucosectomy does not fully eliminate the rectal mucosa [17]. Dysplasia and neoplasia are often found in this area [18, 19]. According to some authors, ileorectal anastomosis should safeguard patients against pouch polyps, dysplasia and neoplasia. This procedure requires the same follow-up endoscopy, offers better quality of life and reduces the risk of temporary ileostomy. Qualification for ileorectal anastomosis is individual [3] and depends also on the amount of rectal polyps—5–10 usually justify this technique [4]. The risk of recurrent polyps may be reduced by Sulindac chemoprevention [20, 21]. Advantages of ileorectal anastomosis emphasized by its advocates are debatable. Functional results and survival rate are nearly the same in patients 5 years after the mentioned procedure and ileal pouch-anal anastomosis [22]. Functioning of patients after restorative proctocolectomy assessed by the SF-36 questionnaire is comparable to that of not operated on [23].
In this study, in patients after restorative proctocolectomy, we diagnosed pouchitis in 20.6% of cases. In our previous survey, the frequency of both FAP and CU pouchitis was nearly the same [24]. According to the recent bibliography, there is a lower percentage of pouchitis in FAP patients—3–14% than in CU individuals—25% [25]. The cause of a high percentage of FAP pouchitis in this study may be the result of its character. Patients with episodes of acute pouchitis and the chronic one visited the out-patient clinic more often. Asymptomatic FAP patients after prophylactic restorative proctocolectomy were more concentrated on the symptoms such as abdominal pain, diarrhoea, bleeding or elevation of fever. These were in turn subjective manifestations suggestive of pouchitis increasing the PDAI score. Numerous groups of FAP patients with pouchitis could be also due to social and economic conditions of these patients: diagnosis of pouchitis, especially chronic, based mainly on symptoms reported in patients could significantly simplify receiving of permanent social and financial benefits. It should be also concerned the high percentage of pouchitis diagnosed with PDAI scale in patients operated because of FAP.
There was no correlation between pouchitis and pouch dysplasia in FAP patients in this study. Pouch dysplasia in CU patients subjected to restorative proctocolectomy is primarily based on pouchitis with coexisting villous atrophy. Pouch dysplasia in FAP patients develops independently of pouchitis. Mutations of APC and MUTYH genes are responsible for a different mechanism of dysplasia formation [26]. Inflammation of the pouch mucosa makes the identification of polyps even more difficult. That is why it was worked out a two-stage formula in patients with severe pouchitis. In this study, it was firstly managed in a standard way. As soon as symptoms disappeared, endoscopic examination was performed in order to find possible foci of dysplasia or neoplasia.
In this material, five cases of neoplasia were found. Three patients in this group refused to be regularly followed-up that proves the worldwide trend of high-grade dysplasia and adenocarcinoma mainly occurring in such individuals [27]. The remaining two FAP patients refused pouch excision at the moment of high-grade dysplasia diagnosis due to not radical polypectomies. Neoplasia appeared 13–16 years after surgery confirmed by the estimated frequency of adenocarcinoma based on the Kaplan–Meier’s curves.
In conclusion, the Polyposis Registry encompassing whole country is the best way of controlling FAP patients. The main clinical problem in FAP patients after restorative proctocolectomy is the occurrence of the dysplasia. The regular lifelong endoscopic monitoring gives the opportunity of the early detection of the dysplasia and can protect against neoplasia [28, 29].
References
European Medicines Agency Doc. Ref.:EMEA/COMP/264/04 [http://www.emea.europa.eu/pdfs/human/comp/opinion/026404en.pdf]
Hassan I, Chua HK, Wolff BG, Donnelly SF, Dozois RR, Larson DR, Schleck CD, Nelson H (2005) Quality of life after ileal pouch-anal anastomosis and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 48(11):2032–2037
Aziz O, Athanasiou T, Fazio VW, Nicholls RJ, Darzi AW, Church J, Phillips RK, Tekkis PP (2006) Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg 93(4):407–417
Valanzano R, Ficari F, Curia MC, Aceto G, Veschi S, Cama A, Battista P, Tonelli F (2007) Balance between endoscopic and genetic information in the choice of ileorectal anastomosis for familial adenomatous polyposis. J Surg Oncol 95(1):28–33
Kartheuser A, Stangherlin P, Brandt D, Remue C, Sempoux C (2006) Restorative proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis revisited. Fam Can 5(3):241–260, discussion 261–2
Galiatsatos P, Foulkes WD (2006) Familial adenomatous polyposis. Am J Gastroenterol 101(2):385–398
Kadmon M (2005) Preventive surgery for familial adenomatous polyposis coli. Chirurg 76(12):1125–1134
Moussata D, Nancey S, Lapalus MG, Prost B, Chavaillon A, Bernard G, Ponchon T, Saurin JC (2008) Frequency and severity of ileal adenomas in familial adenomatous polyposis after colectomy. Endoscopy 40(2):120–125
Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF (1994) Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc 69:409–415
Campos FG, Habr-Gama A, Kiss DR, da Silva EV, Rawet V, Imperiale AR, Perez R, da Silva JH, Sousa AH Jr, Gama-Rodrigues J (2005) Adenocarcinoma after ileoanal anastomosis for familial adenomatous polyposis: review of risk factors and current surveillance apropos of a case. J Gastrointest Surg 9(5):695–702
Tajika M, Nakamura T, Nakahara O, Kawai H, Komori K, Hirai T, Kato T, Bhatia V, Baba H, Yamao K (2009) Prevalence of adenomas and carcinomas in the ileal pouch after proctocolectomy in patients with familial adenomatous polyposis. J Gastrointest Surg 13(7):1266–1273
Friederich P, de Jong AE, Mathus-Vliegen LM, Dekker E, Krieken HH, Dees J, Nagengast FM, Vasen HF (2008) Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 6(11):1237–1242
Parc YR, Olschwang S, Desaint B, Schmitt G, Parc RG, Tiret E (2001) Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg 233(3):360–364
Nilubol N, Scherl E, Bub DS, Gorfine SR, Marion J, Harris MT, Kornbluth A, Lichtiger S, Rubin P, George J, Chapman M, Harpaz N, Present D, Bauer JJ (2007) Mucosal dysplasia in ileal pelvic pouches after restorative proctocolectomy. Dis Colon Rectum 50(6):825–831
Eigenbrod T, Kullmann F, Klebi F (2006) Resection of the small bowel adenocarcinoma liver metastasis combined with neoadjuvant and adjuvant chemotherapy results in extended disease-free period—a case report. Int J Gastrointest Cancer 37(2–3):94–97
Heppell J, Weiland LH, Perrault J, Pemberton JH, Telander RL, Beart RW Jr (1983) Fate of the rectal mucosa after rectal mucosectomy and ileoanal anastomosis. Dis Colon Rectum 26(12):768–771
Walker M, Radley S (2006) Adenocarcinoma in an ileoanal pouch formed for ulcerative colitis in a patient with primary sclerosing cholangitis and a liver transplant: report of a case and review of the literature. Dis Colon Rectum 49(6):909–912
Remzi FH, Fazio VW, Delaney CP, Preen M, Ormsby A, Bast J, O'Riordain MG, Strong SA, Church JM, Petras RE, Gramlich T, Lavery IC (2003) Dysplasia of the anal transitional zone after ileal pouch-anal anastomosis: results of prospective evaluation after a minimum of ten years. Dis Colon Rectum 46(1):6–13
Holder-Murray J, Fichera A (2008) Anal transition zone in the surgical management of ulcerative colitis. World J Gastroenterol 15(7):769–773
Tonelli F, Valanzano R, Messerini L, Ficari F (2000) Long-term treatment with sulindac in familial adenomatous polyposis: is there an actual efficacy in prevention of rectal cancer? J Surg Oncol 74(1):15–20
Filippakis GM, Zografos G, Pararas N, Lanitis S, Georgiadou D, Filippakis MG (2007) Spontaneous regression of rectal polyps following abdominal colectomy and ileorectal anastomosis for familial adenomatous polyposis, without sulindac treatment: report of four cases. Endoscopy 39(7):665–668
Yamaguchi T, Yamamoto S, Fujita S, Akasu T, Moriya Y (2010) Long-term outcome of metachronous rectal cancer following ileorectal anastomosis for familial adenomatous polyposis. J Gastrointest Surg 14(3):500–505
Wuthrich P, Gervaz P, Ambrosetti P, Soravia C, Morel P (2009) Functional outcome and quality of life after restorative proctocolectomy and ileo-anal pouch anastomosis. Swiss Med Wkly 139(13–14):193–197
Banasiewicz T, Walkowiak J, Marciniak R, Krokowicz P, Lisowska A, Grzymisławski M, Drews M (2007) Nasilenie procesu zapalnego błony śluzowej zbiorników jelitowych u chorych z proktokolektomia odtwórczą. Proktologia 8(3–4): 159–166. 25
Yu ED, Shao Z, Shen B (2007) Pouchitis. World J Gastroenterol 13(42):5598–5604
Pezzi A, Roncucci L, Benatti P, Sassatelli R, Varesco L, Di Gregorio C, Venesio T, Pedroni M, Maffei S, Reggiani Bonetti L, Borsi E, Ferrari M, Martella P, Rossi G, Ponz De Leon M (2009) Relative role of APC and MUTYH mutations in the pathogenesis of familial adenomatous polyposis. Scand J Gastroenterol 44(9):1092–1100
Cordero Fernández C, Pizarro Moreno A, Garzón Benavides M, García-Lozano R, Belda Laguna O, Sobrino S, Bozada JM, Zulueta Dorado T (2007) Follow-up after surgical treatment of patients with familial adenomatous polyposis: results in a southern Spanish population. Rev Esp Enferm Dig 99(8):440–445
Olsen KØ, Juul S, Bülow S, Järvinen HJ, Bakka A, Björk J, Oresland T, Laurberg S (2003) Female fecundity before and after operation for familial adenomatous polyposis. Br J Surg 90:227–231. doi:10.1002/bjs.4082
Hurlstone DP, Saunders BP, Church JM (2008) Endoscopic surveillance of the ileoanal pouch following restorative proctocolectomy for familial adenomatous polyposis. Endoscopy 40:437–442. doi:10.1055/s-2007-995655
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Banasiewicz, T., Marciniak, R., Kaczmarek, E. et al. The prognosis of clinical course and the analysis of the frequency of the inflammation and dysplasia in the intestinal J-pouch at the patients after restorative proctocolectomy due to FAP. Int J Colorectal Dis 26, 1197–1203 (2011). https://doi.org/10.1007/s00384-011-1241-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1241-5