Abstract
Purpose
Autophagy is a natural mechanism aimed to degrade and recycle cellular components within cells. Previous studies reported that autophagy in the intestinal epithelium can be activated and that excessive autophagy can have negative consequences. However, the mechanism by which autophagy is regulated during intestinal epithelial injury remains unclear. This study aimed to investigate the mechanism of autophagy regulation during intestinal epithelial cells (IEC) injury.
Methods
Rat IEC18 were exposed to hypoxia and Lipopolysaccharide (LPS) (200 μg/ml) to induce injury. IEC18 were treated with autophagy initiation inhibitor, Wortmannin or with autophagy degradation inhibitor, Bafilomycin A1 were added for 24 h. We assessed the number and diameter of autophagic vacuoles, Cell viability, inflammation and apoptosis.
Results
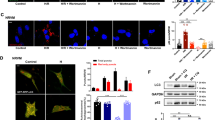
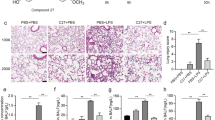
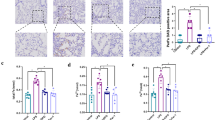
Hypoxia and LPS administration increased the number and diameter of autophagic vacuoles in IEC18. Wortmannin administration reduced the number and diameter of autophagic vacuoles. On the contrary, Bafilomycin A1 administration increased the number of autophagic vacuoles. Cell viability increased following administration of Wortmannin and decreased following administration of Bafilomycin A1.
Conclusions
We found that accumulation of autophagic vacuoles which characterize excessive or incomplete autophagy was detrimental to cell survival. This was shown by an increase in the number and size of the autophagic vacuoles with Bafilomycin A1treatment after hypoxia and LPS stressors relative to hypoxia and LPS alone. Conversely, there was a decrease in the number of autophagic vacuoles with Wortmannin treatment after hypoxia and LPS stressors relative to hypoxia and LPS alone. Therefore, reducing autophagosomes accumulation may represent a novel therapeutic strategy for intestinal injury.


Similar content being viewed by others
References
Choi AM, Ryter SW, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368:651–662
Mizushima N, Noda T, Yoshimori T et al (1998) A protein conjugation system essential for autophagy. Nature 395:395–398
Munson MJ, Ganley IG (2015) MTOR, PIK3C3, and autophagy: Signaling the beginning from the end. Autophagy 11:2375–2376
Boya P, González-Polo RA, Casares N et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040
Song S, Tan J, Miao Y et al (2017) Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J Cell Physiol 232:2977–2984
Hosokawa N, Hara T, Kaizuka T et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20:1981–1991
Takeuchi H, Kondo Y, Fujiwara K et al (2005) Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 65:3336–3346
Ertmer A, Huber V, Gilch S et al (2007) The anticancer drug imatinib induces cellular autophagy. Leukemia 21:936–942
Crazzolara R, Cisterne A, Thien M et al (2009) Potentiating effects of RAD001 (Everolimus) on vincristine therapy in childhood acute lymphoblastic leukemia. Blood 113:3297–3306
Hotchkiss RS, Strasser A, McDunn JE et al (2009) Cell death in disease: mechanisms and emerging therapeutic concepts. N Engl J Med 361:1570–1583
Neal M, Sodhi C, Dyer M et al (2013) A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 190:3541–3551
Arcaro A, Wymann MP (1993) Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296:297–301
Blommaart EF, Krause U, Schellens JP et al (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243:240–246
Mauvezin C, Neufeld TP (2015) Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome–lysosome fusion. Autophagy 11:1437–1438
Button RW, Luo S (2017) The formation of autophagosomes during lysosomal defect: a new source of cytotoxicity. Autophagy 13:1797–1798
Button RW, Roberts SL, Willis TL et al (2017) Accumulation of autophagosomes confers cytotoxicity. J Biol Chem 292:13599–13614
Yu Y, Shiou S, Guo Y et al (2013) Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS One 8:e69620
Li B, Yao X, Luo Y et al (2019) Inhibition of autophagy attenuated intestinal injury after intestinal I/R via mTOR signaling. J Surg Res 243:363–370
Conway KL, Kuballa P, Song JH et al (2013) Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 145:1347–1357
Maynard A, Dvorak K, Khailova L et al (2010) Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299:614–622
Acknowledgements
AP is supported by a Canadian Institutes of Health Research (CIHR) Foundation Grant 353857 and is the Robert M. Filler Chair of Surgery, The Hospital for Sick Children (HSC).
Author information
Authors and Affiliations
Contributions
MY: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. CL, SC, YY, MA, BL: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript. AP: conception and design, financial support, final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamoto, M., Lee, C., Chusilp, S. et al. The role of autophagy in intestinal epithelial injury. Pediatr Surg Int 35, 1389–1394 (2019). https://doi.org/10.1007/s00383-019-04566-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-019-04566-2