Abstract
Background
The intestinal adaptive response [increased epithelial cell (EC) proliferation and apoptosis] after massive small bowel resection (SBR) is partially controlled by intraepithelial lymphocytes (IEL). To identify IEL factors contributing to EC adaptation post-SBR we utilized microarray assays.
Methods
Mice underwent a 70% SBR (SBR1w/SBR4w) or sham operation (Sham1w/Sham4w). After 1 or 4 weeks (1w, 4w) small bowel was harvested, and IEL isolated. Determination of the EC-proliferation rate used BrdU incorporation, and of the EC-apoptotic rate used Annexin V staining. Affymetrix system microarrays (12,491 genes) were performed to examine IEL-mRNA expression. Results were considered significant if fold-change (FC) between groups was >2 and P <0.05 (F-test), or FC>3 and 0.05> P >0.01, or FC>4 and P >0.05. Significant genes were confirmed by conventional RT-PCR.
Results
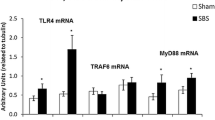
The SBR EC-proliferation rate increased significantly in both 1w and 4w groups compared to Sham: SBR1w 0.24±0.07 vs. Sham1w 0.12±0.02 ( P =0.03); SBR4w 0.35±0.04 vs. Sham4w 0.19±0.02 ( P <0.01). The EC-apoptotic rate was unchanged in the 1w group, but significantly differed from controls after 4 weeks: SBR4w 39.92±6.78 vs. Sham4w 12.56±6.44 ( P <0.01). Microarray results were analyzed to identify potential growth-modifying IEL genes. The following were identified (function in parenthesis; A, apoptosis; P, proliferation): lipocalin 2 (promotes A), angiotensin converting enzyme (increases A), Rap2 interacting protein (reduces A, promotes P), amphiregulin (promotes P) and leucine-rich-α2-glycoprotein (promotes A, reduces P). Based on RT-PCR results these genes showed significant changes between groups. The increase in ACE at 1w preceded the observed apoptotic changes. The alterations in lipocalin 2, Rap2 and amphiregulin at 4w coincided with the marked changes in growth and apoptosis in the SBR mice.
Conclusions
IEL undergo temporal changes after SBR. These findings provide profound insight into potential IEL-dependent regulation of EC homeostasis post-SBR.


Similar content being viewed by others
References
Beagley KW, Husband AJ (1998) Intraepithelial lymphocytes: origins, distribution, and function. Crit Rev Immunol 18:237–254
Guy-Grand D, Di Santo JP, Henchoz P, Malassis-Seris M, Vassalli P (1998) Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol 28:730–744
Eisenbraun MD, Mosley RL, Teitelbaum DH, Miller RA (2000) Altered development of intestinal intraepithelial lymphocytes in p-glycoprotein-deficient mice. Dev Comp Immunol 24:783–795
Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW (1996) Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 183:441–449
Kiristioglu I, Teitelbaum DH (1998) Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res 79:91–96
Welters CF, Dejong CH, Deutz NE, Heineman E (2002) Intestinal adaptation in short bowel syndrome. ANZ J Surg 72:229–236
Falcone RA Jr, Stern LE, Kemp CJ, Shin CE, Erwin CR, Warner BW (1999) Apoptosis and the pattern of DNase I expression following massive small bowel resection. J Surg Res 84:218–222
Klein RM, McKenzie JC (1983) The role of cell renewal in the ontogeny of the intestine. J Pediatr Gastroenterol Nutr 2:204–228
Iwakiri D, Podolsky DK (2001) Keratinocyte growth factor promotes goblet cell differentiation through regulation of goblet cell silencer inhibitor. Gastroenterology 120:1372–1380
Boismenu R, Havran WL (1994) Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science 266:1253–1255
Barrett T, Gajewski T, Danielpour D, Chang E, Beagley K, Bluestone J (1992) Differential function of intestinal intraepithelial lymphocyte subsets. J Immunol 149:1124–1130
Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR (1999) Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol 276: G599–605
Flower DR (1996) The lipocalin protein family: structure and function. Biochem J 318:1–14
Devireddy LR, Teodoro JG, Richard FA, Green MR (2001) Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 293:829–834
Janoueix-Lerosey I, Pasheva E, de Tand MF, Tavitian A, de Gunzburg J (1998) Identification of a specific effector of the small GTP-binding protein Rap2. Eur J Biochem 252:290–298
Janda E, Litos G, Grunert S, Downward J, Beug H (2002) Oncogenic Ras/Her-2 mediate hyperproliferation of polarized epithelial cells in 3D cultures and rapid tumor growth via the PI3 K pathway. Oncogene 21:5148–5159
Danielsen AJ, Maihle NJ (2002) The EGF/ErbB receptor family and apoptosis. Growth Factors 20:1–15
Nancy V, Wolthuis RM, de Tand MF, Janoueix-Lerosey I, Bos JL, de Gunzburg J (1999) Identification and characterization of potential effector molecules of the Ras-related GTPase Rap2. J Biol Chem 274:8737–8745
Jimenez B, Pizon V, Lerosey I, Beranger F, Tavitian A, de Gunzburg J (1991) Effects of the Ras-related Rap2 protein on cellular proliferation. Int J Cancer 49:471–479
Sehgal I, Bailey J, Hitzemann K, Pittelkow MR, Maihle NJ (1994) Epidermal growth factor receptor-dependent stimulation of amphiregulin expression in androgen-stimulated human prostate cancer cells. Mol Biol Cell 5:339–347
Li S, Plowman GD, Buckley SD, Shipley GD (1992) Heparin inhibition of autonomous growth implicates amphiregulin as an autocrine growth factor for normal human mammary epithelial cells. J Cell Physiol 153:103–111
Johnson GR, Saeki T, Gordon AW, Shoyab M, Salomon DS, Stromberg K (1992) Autocrine action of amphiregulin in a colon carcinoma cell line and immunocytochemical localization of amphiregulin in human colon. J Cell Biol 118:741–751
Silvy M, Giusti C, Martin PM, Berthois Y (2001) Differential regulation of cell proliferation and protease secretion by epidermal growth factor and amphiregulin in tumoral versus normal breast epithelial cells. Br J Cancer 84:936–945
Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC (1999) Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126:2739–2750
Dunker N, Schmitt K, Schuster N, Krieglstein K (2002) The role of transforming growth factor beta-2, beta-3 in mediating apoptosis in the murine intestinal mucosa. Gastroenterology 122:1364–1375
Dignass AU, Sturm A (2001) Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol 13:763–770
Wang R, Zagariya A, Ibarra-Sunga O, Gidea C, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal BD (1999) Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 276:L885–889
Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P (1997) Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 29:859–870
Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM (1997) Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res 81:970–976
Schindler R, Dinarello CA, Koch KM (1995) Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and interleukin 1 by human peripheral blood mononuclear cells. Cytokine 7:526–533
Constantinescu CS, Goodman DB, Ventura ES (1998) Captopril and lisinopril suppress production of interleukin-12 by human peripheral blood mononuclear cells. Immunol Lett 62:25–31
Kono Y, Sawada S, Kawahara T, Tsuda Y, Higaki T, Yamasaki S, Imamura H, Tada Y, Sato T, Hiranuma O, Akamatsu N, Komatsu S, Tamagaki T, Nakagawa K, Tsuji H, Nakagawa M (2002) Bradykinin inhibits serum-depletion-induced apoptosis of human vascular endothelial cells by inducing nitric oxide via calcium ion kinetics. J Cardiovasc Pharmacol 39:251–261
Yoshida H, Zhang JJ, Chao L, Chao J (2000) Kallikrein gene delivery attenuates myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Hypertension 35:25–31
Uhal BD, Gidea C, Bargout R, Bifero A, Ibarra-Sunga O, Papp M, Flynn K, Filippatos G (1998) Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol 275:L1013–1017
Odaka C, Mizuochi T (2000) Angiotensin-converting enzyme inhibitor captopril prevents activation-induced apoptosis by interfering with T cell activation signals. Clin Exp Immunol 121:515–522
Yu G, Liang X, Xie X, Su M, Zhao S (2001) Diverse effects of chronic treatment with losartan, fosinopril, and amlodipine on apoptosis, angiotensin II in the left ventricle of hypertensive rats. Int J Cardiol 81:123–129
Acknowledgements
This work was supported by a grant from the National Institute of Health, USA (AI44076–01), by fellowships from Novartis Stiftung Schweiz, Swiss National Foundation, and the Swiss Society of Pediatric Surgery. Microarray analysis was supported through the University of Michigan NIDDK Biotechnology Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wildhaber, B.E., Yang, H., Coran, A.G. et al. Gene alteration of intestinal intraepithelial lymphocytes in response to massive small bowel resection. Ped Surgery Int 19, 310–315 (2003). https://doi.org/10.1007/s00383-003-1001-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-003-1001-x