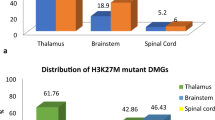
Abstract
Purpose
Diffuse Midline Glioma (DMG) with H3K27M mutation is a rare and aggressive midline high grade glioma with a predominant astrocytic differentiation and K27M mutation in either H3F3A or HIST1H3B/C. This tumor is more common in children than in adults. The current study was aimed to determine clinicohistoradiological and surgical outcome of patients who have undergone surgery for DMG and study disease severity of patients with DMG.
Methods
This is an observational study in which 29 DMG patients were evaluated for clinicohistoradiological and surgical outcomes by assessing the pre and postoperative neurological status.
Result
Survival duration was significantly high in patients with age > 18 years (p = 0.02). Patients who had undergone Radiation Therapy showed higher survival rate (p = 0.05) and the cases with low levels of Ki 67 index had improved post operative outcome (p = 0.002).
Conclusion
DMG with H3K27M mutation in newly classified Central Nervous System tumor are WHO grade IV Tumors, comprising H3K27M mutation as molecular marker for diagnosis and related with a poor prognosis.
Similar content being viewed by others
References
Katherine E. Warren (2012) Diffuse intrinsic pontine glioma: poised for progress. Front Oncol
Taylor KR (2014) Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46:457–461
Wu G (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251
Wu G (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450
Schwartzentruber J (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Natures 482:226–231
Da KQ (2012) K27M mutation in histone H3.3 defines clinically andbiologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124
Lu VM.(2018) Impact of theH3K27M mutation on survival in pediatric high-grade glioma: asystematic review and meta-analysis. J Neurosurgery Pedia. 1:1–9
López G (2017) Diffuse non-midline glioma with H3F3A K27M mutation: a prognostic and treatment dilemma. Acta Neuropathol
Piunti A (2017) Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat Med 23:493–500
Grasso et al (2015) Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 21:827
Morales La Madrid AH (2015) Future clinical trialsin DIPG: bringing epigenetics to the clinic. Front Oncol 2015
Silveira AB (2019) H3.3 K27M depletion increases differentiation and extends latency of diffuse intrinsic pontine glioma growth in vivo [published correction appears in Acta Neuropathol. Acta Neuropathol 137(4):637–655
Bender S (2013) Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24:660–672
Capper D (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474
National Cancer Institute. Available from: https://www.cancer.gov/rare-brain-spine-tumor/tumors/diffuse-midline-gliomas
Gallitto MLS (2019) Role of Radiation Therapy in the Management of Diffuse Intrinsic Pontine Glioma: A Systematic Review. Adv Rad Oncol p. 520–531
Abe H, Natsumeda M, Kanemaru Y, Watanabe J, Tsukamoto Y, Okada M, Yoshimura J, Oishi M, Fujii Y (2018) MGMT Expression Contributes to Temozolomide Resistance in H3K27M-Mutant Diffuse Midline Gliomas and MGMT Silencing to Temozolomide Sensitivity in IDH-Mutant Gliomas. Neurol Med Chir (Tokyo) 58(7):290–295. https://doi.org/10.2176/nmc.ra.2018-0044. Epub 2018 May 31
Gupta N. Brain center tumouruscf. Available from: https://braintumorcenter.ucsf.edu/condition/diffuse-midline-glioma,
Dufour C, Perbet R, Leblond P, Vasseur R, Stechly L (2019) Identification of prognostic markers in diffuse midline gliomas H3K27M mutant. Brain Pathol
Peter W. Lewis, Manuel M. Müller, Matthew S. Koletsky, Francisco Cordero, Shu Lin, Laura A. Banaszynski, Benjamin A. Garcia, Tom W. Muir, Oren J. Becher, And C. David Allis(2013) Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340:857–861,
Toshiyuki EM (2020) Midline Glioma in Adults:Clinicopathological,Genetic, and Epigenetic Analysis. Neurol Med Chir (Tokyo) 60:136–146
Ernst T (2012) Inactivation of polycomb repressive complex components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Europe PMC 119:1208–1213
Nikoloski G. (2010)Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. .42:665–66
Ntziachristos P. (.2012)Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med .18:298–301
Stransky N. (2011)The mutational landscape of head and neck squamous cell carcinoma. Science. 333:1157–1160
Zhang J. (2012)The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature.481:157–163
Haihui Jiang KY (2020) Diffuse midline glioma with H3 K27M mutation: acomparison integrating the clinical, radiological, and molecular features between adult andpediatric patients. Neuro Oncol
Lim JX (2020) H3K27M-mutant diffuse midline glioma presenting as synchronouslesions involving pineal and suprasellar region: A case reportlesions involving pineal and suprasellar region: A case reportlesions involving pineal and suprasellar region: A case report. Singapore: National Neuroscience Institute, Department of Neurosurgery
Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N (2015) Diffuse Midline Gliomas with Histone H3-K27M Mutation:A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol
Korones DN (2017) Treatment of newly diagnosed diffuse brain stem gliomas in children: in search ofthe holy grail. Expert Rev Anticancer Ther
Enomoto et al (2020) Neurol Med Chir (Tokyo) 60(3):136–146
Schreck KC, Ranjan S, Skorupan N, Bettegowda C, Eberhart CG, Ames HM, Holdhoff M (2019) Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographicallydetermined midline gliomas. J Neurooncol 143:87–93. https://doi.org/10.1007/s11060-019-03134-x
Cohen KJ, Jabado N, Grill J (2017) (Diffuse intrinsic pontine gliomas—current management and newbiologic insights. Is there a glimmer of hope). Neuro Oncol 19(8):1025–1034
Elena V Daoud, Veena Rajaram, Chunyu Cai, Robert J Oberle, Gregory R Martin, Jack M Raisanen, Charles L White, III, Chan Foong, Bruce E Mickey, Edward Pan, Kimmo J Hatanpaa,(2018) Adult Brainstem Gliomas WithH3K27M Mutation: Radiology, Pathology, and Prognosis. J Neuropathol Exp Neurol 77(4):302–31
Acknowledgements
We are thankful for the support and assistance received from the faculty, residents, technical and non-technical staff members of Department of Neurosurgery, Nizam’s Institute of Medical Sciences, Hyderabad.
Author information
Authors and Affiliations
Contributions
Arvind Kumar: Conception, Organization, Execution, Design, Execution, Writing of the first draft; Suchanda Bhattacharjee: Conception, Organization, Execution, Design, Execution, Review, and critique; Megha S Uppin: Conception, Execution, Review, and critique; Syed Tazeem Fathima: Design, Execution, Review, and critique.
Corresponding author
Ethics declarations
Ethical compliance statement
All procedures performed in study involving human participants was in accordance with the ethical standards of institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Ethics Committee of Nizam’s Institute of Medical Sciences, Hyderabad, India (EC/NIMS/2466/2019).
Informed consent
Informed consent was obtained from all the study participants. We confirm that we have read the Journal’s position on issues.
Data transparency
The authors declare that they comply with field standards.
Competing interest
The authors declare that there is no competing of interest relevant to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar Suman, A., Bhattacharjee, S., Uppin, M.S. et al. Clinicohistoradiological and surgical outcome in diffuse midline glioma. Childs Nerv Syst 40, 65–71 (2024). https://doi.org/10.1007/s00381-023-06095-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06095-9