Abstract
Introduction
Diffuse hemispheric glioma, H3 G34-mutant (DHGs), is a newly categorized tumor in pediatric-type diffuse high-grade gliomas, World Health Organization grade 4, with a poor prognosis. Although prognostic factors associated with genetic abnormalities have been reported, few reports have examined the clinical presentation of DHGs, especially from the viewpoint of imaging findings. In this study, we investigated the relationship between clinical factors, including imaging findings, and prognosis in patients with DHGs.
Methods
We searched Medline through the PubMed database using two search terms: “G34” and “glioma”, between 1 April 2012 and 1 July 2023. We retrieved articles that described imaging findings and overall survival (OS), and added one DHG case from our institution. We defined midline invasion (MI) as invasion to the contralateral cerebrum, brainstem, corpus callosum, thalamus, and basal ganglia on magnetic resonance imaging. The primary outcome was 12-month survival, estimated using Kaplan–Meier curves and logistic regression.
Results
A total of 96 patients were included in this study. The median age was 22 years, and the proportion of male patients was 48.4%. Lesions were most frequently located in the frontal lobe (52.6%). MI was positive in 39.6% of all patients. The median OS was 14.4 months. Univariate logistic regression analysis revealed that OS was significantly worse in the MI-positive group compared with the MI-negative group. Multivariate logistic regression analysis revealed that MI was an independent prognostic factor in DHGs.
Conclusions
In this study, MI-positive cases had a worse prognosis compared with MI-negative cases.
Previous presentations
No portion of this study has been presented or published previously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse hemispheric gliomas, H3 G34-mutant (DHGs), are a newly categorized tumor in pediatric-type diffuse high-grade gliomas in the 2021 World Health Organization classification, with a poor prognosis [1, 2]. DHGs are caused by an amino acid substitution in the histone gene H3F3A (H3.3) in which glycine at position 34 is replaced by arginine or rarely valine (G34R/V) [3,4,5]. Recent studies have shown that the majority of DHGs have ATRX and TP53 alterations, and approximately half have PDGFRA abnormalities [6]. Additionally, most have MGMT promotor methylation [4, 7]. These tumors are most commonly located in the cerebral hemispheres, particularly in the frontoparietal lobes [8]. Histologically, the majority present as high-grade gliomas, such as glioblastomas (GBMs) or anaplastic astrocytomas, while others present as primitive neuroectodermal tumors or low-grade gliomas, pathologically [6, 9,10,11].
Because DHGs are reported to occur in less than 1% of all gliomas, previous studies have involved small sample sizes. Thus, prognostic factors have not been well investigated; however, the prognosis of DHGs is dismal. Recently, Crowell et al. performed a systematic review of 135 patients with DHGs, and univariate analysis showed that age and degree of resection were significantly associated with overall survival (OS) [6]. Other researchers showed that G34V-mutant tumors had significantly worse OS compared with G34R-mutant tumors [12]. These studies evaluated the prognostic impact of clinical factors and genetic alterations, but the impact of radiographic features has not been well investigated. Only one report showed that ill-defined DHG margins were associated with a worse prognosis compared with well-defined margins [8]. Therefore, the purpose of the present study was to clarify the relationship between imaging findings and prognosis in patients with DHGs.
Methods
This study was a non-registered individual participant data review. No protocol was prepared.
Patient cohort
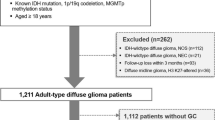
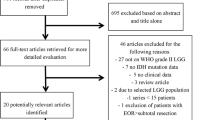
We searched Medline through the PubMed database using two search terms, “G34” and “glioma”, between 1 April 2012 and 1 July 2023. In the present cohort, two neurosurgeons independently conducted the search and selected the cases in August 2023. Among the identified studies, we excluded studies that did not report the tumor location or OS. Studies containing cases duplicated in different published articles were also excluded. There were no restrictions on race or age (Fig. 1).
Additionally, after obtaining institutional review board approval (1608-026) from Okayama University Hospital, we retrospectively reviewed our medical records for cases. We performed immunohistochemistry for patients diagnosed as having glioma in our institute between January 2000 and May 2022 using rabbit monoclonal recombinant antibodies to histone H3.3 G34R or G34V (RevMAb BioSciences, San Francisco, CA, USA; 1:250 dilution for G34R and G34V).
Data extraction
Data were extracted from all available sources in the selected manuscripts and our medical records. Patient demographics and radiographic characteristics comprised age, sex, lesion location, and imaging findings (contrast enhancement, necrosis, cyst formation, apparent diffusion coefficient (ADC) value). Molecular findings, such as H3.3 G34, MGMT, ATRX status and patient outcomes, and surgical details, were also collected.
Midline invasion (MI) was defined as positive if lesions involved the contralateral cerebrum, brainstem, corpus callosum, thalamus, or basal ganglia on either non-contrast-enhanced or contrast-enhanced magnetic resonance imaging. To determine midline invasion, we reviewed only the initial upfront MRI to avoid post-treatment changes. Although ADC values would be valuable to differentiate edema from tumor infiltration, only 25 of 96 patients have the data of ADC values. Therefore we could not use the ADC value secondarily to determine the presence of hypercellular tumor with midline invasion or edema crossing the midline.
Patients were followed from the date of the diagnosis until the date of death or the end of the study period, whichever occurred first. In the present study, patients who were alive at the end of the study were censored only because no patients were lost to follow-up. The primary outcome was defined as OS at 12 months.
Statistical analysis
Continuous variables were expressed as median (interquartile range), and categorical variables were expressed as n (%). We used a t-test to compare the continuous variables of two samples and used the chi-square test to compare categorical variables. Survival probabilities were estimated using the Kaplan–Meier method, and the log-rank test was used to compare the survival distributions between groups, as follows: age (≤ 19 years, 20–29 years, and ≥ 30 years), sex, laterality (right, left, bilateral), MI (positive, negative), G34 status (G34R-positive, G34V-positive), MGMT promoter status (unmethylated, methylated), contrast enhancement (positive, negative), and extent of resection (biopsy, subtotal resection, gross total resection (GTR)). Additionally, using a logistic regression model, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each item using 12-month survival as the outcome. The variables in the multivariate logistic regression model comprised age, sex, MI, and G34 status on the basis of clinical knowledge and previous reports [12]. Extent of resection was excluded owing to its strong correlation with MI (multicollinearity). p < 0.05 was considered statistically significant, and all analyses were performed using GraphPad Prism (version 9.00 for Windows; GraphPad Software, La Jolla, CA, USA).
Results
Of the 67 identified studies, 95 cases in 14 articles [7, 8, 13,14,15,16,17,18,19,20,21,22,23,24] met the inclusion criteria of reporting the tumor location and OS. Additionally, we included one patient from our institute whose diagnosis was confirmed by both immunohistochemistry and genome-wide DNA methylation profiling (Table 1; Fig. 2, Supplementary Fig. 1). The patients’ demographic data are summarized in Table 1. Forty-three of the 96 patients (44.8%) were male, and the median age was 22 years (whole range, 8–66 years) (interquartile range, 18–29), which was older than previous reports [6]. The data except for age, sex, and MI included missing data (Table 1). The neurological findings were shown in supplementary Table 1.
Radiographic findings
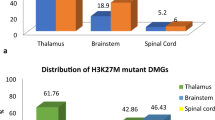
An ipsilateral lesion was observed in 81.5% (44/54) of the patients, and the left hemisphere was slightly dominant (27/54 cases, 50.0%). Bilateral lesions were observed in 18.5% (10/54) of the patients. Consistent with previous reports [6, 18], tumors were located most often in the frontal lobe (50/95cases, 52.6%). Contrast enhancement was seen in 62.3% (48/77) of the patients, necrosis in 30.6% (11/36), and cyst formation in 48.5% (16/33). A low ADC value was observed in 96.0% (24/25) of the cases. MI was observed in 39.6% (38/96) of the cases, namely 18 in the corpus callosum, 13 in the basal ganglia, 10 in the contralateral cerebrum, 10 in the thalamus, and 4 in the brainstem (some patients had lesions in more than one location).
Genetic alterations
Regarding the missense mutation in the H3-3 A gene, p.G34R was observed in 88.6% (70/79) of the cases, whereas p.G34V was observed in 11.4% (9/79). Additionally, 97.3% (36/37) of the cases had alterations in the ATRX gene. MGMT promoter methylation was identified in 89.1% (57/64) of the cases.
Outcomes
Of the 83 patients who underwent surgery, biopsy results were reported in 25.3% (21/83) of the cases; subtotal resection results were reported in 32.5% (27/83), and GTR results were reported in 42.2% (35/83) (Table 1). The median OS of all cases was 14.4 months, and the 12- and 24-month survival rates were 68.1% and 42.0%, respectively.
MI predicts the prognosis of DHGs, H3 G34-mutant
Figure 3 shows the OS curves for each clinical, radiographic, and genetic feature. In the univariate analysis, compared with the MI-negative group, OS was significantly shorter in the MI-positive group (p = 0.0135) and significantly longer in the GTR-achieved vs. other two group (p < 0.0001).
Kaplan–Meier survival curve demonstrating overall survival. Overall survival was evaluated with (A) age, (B) sex, (C) laterality, (D) midline invasion (MI), (E) genetic alteration in H3F3A, (F) methylation status of the MGMTMGMT promoter, (G) contrast enhancement (CE), and (H) extent of resection at the primary surgery. STR: subtotal resection, GTR: gross total resection
Univariate logistic regression analysis with 12-month OS as the outcome showed that the MI-positive group had a significantly higher mortality than that in the MI-negative group (OR = 4.48, 95% CI = 1.70–12.4; p < 0.01), and the GTR-achieved group had a significantly lower mortality than that in the group that underwent biopsy alone (OR = 0.14, 95% CI = 0.03–0.57; p = 0.01) (Table 2).
Furthermore, multivariate logistic regression was performed to examine the association of MI with 12-month OS as the outcome. Compared with the MI-negative group, the MI-positive group had significantly higher mortality after 12 months (OR = 3.60, 95% CI = 1.20–11.5; p = 0.02) (Table 3).
Discussion
DHGs were a newly defined tumor entity in the World Health Organization classification 5th edition, and were reported to occur in less than 1% of all gliomas [1]. Yoshimoto et al. reported that in their analysis of 411 gliomas, 4 tumors had G34R mutations [13]. In contrast, more than 30% of high-grade gliomas in adolescents and young adults harbor heterozygous mutations in the non-canonical H3.3 variant, resulting in glycine 34 to arginine or valine (G34R/V) amino acid substitution [25]. DHGs exhibit primitive neuroectodermal tumor-like or GBM-like histology and almost invariably carry ATRX and TP53 mutations, and lack immunoreactivity for OLIG2 [18]. Additionally, DHGs frequently exhibit MGMT promoter methylation and lack TERT promoter mutations [18].
Although DHGs were first reported in 2012, precise treatment and prognostic factors have not been well elucidated [3, 4]. Regarding treatment, a systematic review showed that most cases underwent upfront surgical resection; however, GTR was achieved in less than 50% of the cases [6]. Radiotherapy and chemotherapy have also been used as initial treatment, but the details have not been well described. Of the 31 patients in this study with detailed chemotherapy regimen information, 20 (64%) received temozolomide-based therapy [6]. Although the significance of MGMT promoter methylation in DHGs has not been investigated, Crowell et al. showed that patients harboring MGMT promoter methylation showed superior survival [6]. Vuong et al. reported that PDGFRA and EGFR amplification had a negative prognostic impact, with the G34V genotype having a worse prognosis than that for G34R [12]. In their review, methylation of the MGMT promoter was also observed in most DHGs. Furthermore, G34V-positive DHGs tended to have a worse prognosis than G34R-positive DHGs.
In this study, we investigated the radiographic and genetic factors related to prognosis in patients with DHGs. To the best of our knowledge, this is the largest study to have analyzed the radiographic features of DHGs. Korshunov et al. investigated 81 patients with DHGs and reported that 80% of the tumors were located in the temporal and parietal lobes [26]. In our study, the most common tumor location was the frontal lobe, but many tumors also invaded the parietal and temporal lobes. Additionally, DHGs were characterized by slight gadolinium contrast enhancement, peritumoral edema on T2-weighted/fluid-attenuated inversion recovery imaging, and hyperintensity on diffusion-weighted imaging or a low ADC value [16]. In the present study, 94% of the cases had a low ADC value, which can be a characteristic imaging finding. According to Ohnishi et al., diffusion-weighted imaging hyperintensity and a low ADC value may be associated with high cellularity in DHGs [16].
Previous studies have shown that deep supratentorial extension involving the thalamus, basal ganglia, and corpus callosum in GBMs is associated with a poor prognosis [27]. According to Dayani et al., GBM spread through the corpus callosum to the contralateral cerebrum (“butterfly GBM”) was associated with a worse prognosis than that for localized GBMs, with a median overall survival of 3.2 months [28]. Although radiographic features, such as hemispheric location, are diagnostic criteria for DHGs, the relationship between imaging findings and prognosis has not been well investigated. Only one study revealed that OS for DHGs with ill-defined margins was significantly lower than that for DHGs with well-defined margins [8]. Our study summarized the clinical and radiological findings of 93 DHG cases, and MI was identified as a prognostic factor.
There is no difference in prognosis if GTR can be achieved, even in cases with tumor invasion into deep structures, in patients with GBMs [27]. Because tumor invasion into deep structures interferes with GTR, the addition of adjuvant therapy fails to suppress the growth of residual tumor, resulting in short OS. In the present study, the DHG MI-negative group had better 12-month OS compared with the MI-positive group. However, univariate analysis suggested that the resection rate was strongly correlated with OS. In this study, we analyzed MI as a prognostic factor; however, the surgical technique may be the most important factor because surgical strategies and resection rates are determined on the basis of imaging findings. Thus, safe GTR may prolong OS in MI-negative patients with DHGs. Recently, there have also been reports of using language mapping and 5-aminolevulinic acid during surgery for DHGs [24]. Combining multiple modalities, such as tractography, the use of navigation systems and intraoperative magnetic resonance imaging, and awake surgery, which are usually used in glioblastoma surgery [29], enables safe and maximum resection of DHGs.
Although the presence or absence of MI at the start of treatment can be a useful finding in predicting prognosis, even in MI-positive cases, maximal resection while preserving function, as in conventional GBM treatment, may improve the prognosis of DHG patients.
Study limitations
This study is limited by the retrospective design and small sample size. Additionally, there were cases with no confirmed diagnosis, unreported cases, and cases with missing data including tumor size, which were not analyzed, resulting in a high degree of selection bias. Furthermore, the results may be biased because the effects of unmeasured confounders cannot be accounted for. Finally, image interpretation methods and surgical strategies varied between institutions. Future large-scale studies are needed.
Conclusions
Similar to GBMs, DHGs have a poor prognosis if they invade deep structures or the contralateral cerebrum; however, safe and maximal tumor resection may prolong survival.
Data availability
No datasets were generated or analysed during the current study.
References
The WHO Classification of Tumours Editorial Board (2021) Central nervous system tumours. Lyon, France
Otani Y, Satomi K, Suruga Y, Ishida J, Fujii K, Ichimura K, Date I (2023) Utility of genome-wide DNA methylation profiling for pediatric-type diffuse gliomas. Brain Tumor Pathol 40(2):56–65. https://doi.org/10.1007/s10014-023-00457-6
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jäger N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Frühwald MC, Roggendorf W, Kramm C, Dürken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231. https://doi.org/10.1038/nature10833
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Frühwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Dürken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437. https://doi.org/10.1016/j.ccr.2012.08.024
Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Baker SJ, Project SJCsRHWUPCG (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253. https://doi.org/10.1038/ng.1102
Crowell C, Mata-Mbemba D, Bennett J, Matheson K, Mackley M, Perreault S, Erker C (2022) Systematic review of diffuse hemispheric glioma, H3 G34-mutant: outcomes and associated clinical factors. Neurooncol Adv 4:vdac133. https://doi.org/10.1093/noajnl/vdac133
Lim KY, Won JK, Park CK, Kim SK, Choi SH, Kim T, Yun H, Park SH (2021) H3 G34-mutant high-grade glioma. Brain Tumor Pathol 38:4–13. https://doi.org/10.1007/s10014-020-00378-8
Kurokawa R, Baba A, Kurokawa M, Pinarbasi ES, Makise N, Ota Y, Kim J, Srinivasan A, Moritani T (2022) Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: a case series and systematic review. J Neuroimaging 32:17–27. https://doi.org/10.1111/jon.12939
Neumann JE, Dorostkar MM, Korshunov A, Mawrin C, Koch A, Giese A, Schüller U (2016) Distinct histomorphology in Molecular subgroups of Glioblastomas in Young patients. J Neuropathol Exp Neurol 75:408–414. https://doi.org/10.1093/jnen/nlw015
Gessi M, Gielen GH, Hammes J, Dörner E, Mühlen AZ, Waha A, Pietsch T (2013) H3.3 G34R mutations in pediatric primitive neuroectodermal tumors of central nervous system (CNS-PNET) and pediatric glioblastomas: possible diagnostic and therapeutic implications? J Neurooncol 112:67–72. https://doi.org/10.1007/s11060-012-1040-z
Oliveira VF, De Sousa GR, Dos Santos AC, Saggioro FP, Machado HR, de Oliveira RS, Tone LG, Valera ET (2021) Evaluating H3F3A K27M and G34R/V somatic mutations in a cohort of pediatric brain tumors of different and rare histologies. Childs Nerv Syst 37:375–382. https://doi.org/10.1007/s00381-020-04852-8
Vuong HG, Le HT, Dunn IF (2022) The prognostic significance of further genotyping H3G34 diffuse hemispheric gliomas. Cancer 128:1907–1912. https://doi.org/10.1002/cncr.34156
Yoshimoto K, Hatae R, Sangatsuda Y, Suzuki SO, Hata N, Akagi Y, Kuga D, Hideki M, Yamashita K, Togao O, Hiwatashi A, Iwaki T, Mizoguchi M, Iihara K (2017) Prevalence and clinicopathological features of H3.3 G34-mutant high-grade gliomas: a retrospective study of 411 consecutive glioma cases in a single institution. Brain Tumor Pathol 34:103–112. https://doi.org/10.1007/s10014-017-0287-7
Andreiuolo F, Lisner T, Zlocha J, Kramm C, Koch A, Bison B, Gareton A, Zanello M, Waha A, Varlet P, Pietsch T (2019) H3F3A-G34R mutant high grade neuroepithelial neoplasms with glial and dysplastic ganglion cell components. Acta Neuropathol Commun 7:78. https://doi.org/10.1186/s40478-019-0731-5
Cheng Y, Bao W, Wu Q (2020) Cerebral hemispheric glioblastoma with PNET-like morphology and histone H3.3 G34 mutation in younger patients: report of three rare cases and diagnostic pitfalls. Indian J Pathol Microbiol 63:262–266. https://doi.org/10.4103/IJPM.IJPM_544_19
Onishi S, Amatya VJ, Karlowee V, Takeshima Y, Sugiyama K, Kurisu K, Yamasaki F (2020) Radiological and immunostaining characteristics of H3.3 G34R-Mutant glioma: a report of 3 cases and review of the literature. Pediatr Neurosurg 55:319–325. https://doi.org/10.1159/000511672
Wood MD, Neff T, Nickerson JP, Sayama C, Raslan AM, Ambady P, Corless CL, Nazemi KJ (2021) Post-treatment hypermutation in a recurrent diffuse glioma with H3.3 p.G34 mutation. Neuropathol Appl Neurobiol 47:460–463. https://doi.org/10.1111/nan.12679
Picart T, Barritault M, Poncet D, Berner LP, Izquierdo C, Tabouret E, Figarella-Branger D, Idbaïh A, Bielle F, Bourg V, Vandenbos FB, Moyal EC, Uro-Coste E, Guyotat J, Honnorat J, Gabut M, Meyronet D, Ducray F (2021) Characteristics of diffuse hemispheric gliomas, H3 G34-mutant in adults. Neurooncol Adv 3:vdab061. https://doi.org/10.1093/noajnl/vdab061
Hu W, Duan H, Zhong S, Zeng J, Mou Y (2022) High frequency of PDGFRA and MUC family gene mutations in diffuse hemispheric glioma, H3 G34-mutant: a glimmer of hope? J Transl Med 20:64. https://doi.org/10.1186/s12967-022-03258-1
Wang L, Shao L, Li H, Yao K, Duan Z, Zhi C, Song S, Cheng Y, Wang F, Wang W, Piao Y, Gui Q, Lu D, Qi X, Teng L (2022) Histone H3.3 G34-mutant diffuse gliomas in adults. Am J Surg Pathol 46:249–257. https://doi.org/10.1097/PAS.0000000000001781
Yamada CAF, Soldatelli MD, do Amaral LLF, Campos CMS, de Moraes PL, Chaddad-Neto FEA, Lancellotti CLP (2023) Case Report: Evolutionary Clinical-Radiological features of a diffuse Hemispheric Glioma, H3 G34 mutant with over 5 years of Survival. Case Rep Oncol 16:279–286. https://doi.org/10.1159/000530181
Kitakami K, Beppu T, Sato Y, Kurose A, Ogasawara K (2023) Utility of arterial spin labeling for objective assessment of intratumoral microvessels in diffuse hemispheric glioma, H3 G34R-mutant: a case report and literature review. Radiol Case Rep 18:856–861. https://doi.org/10.1016/j.radcr.2022.11.074
Kalelioglu T, Emerson D, Luk A, Lopes B, Patel SH (2023) Imaging features of diffuse hemispheric glioma, H3 G34-mutant: report of 4 cases. J Neuroradiol 50:309–314. https://doi.org/10.1016/j.neurad.2022.12.001
Lavrador JP, Reisz Z, Sibtain N, Rajwani K, Baig Mirza A, Vergani F, Gullan R, Bhangoo R, Ashkan K, Bleil C, Zebian B, Clark B, Laxton R, King A, Bodi I, Al-Saraj S (2023) H3 G34-mutant high-grade gliomas: integrated clinical, imaging and pathological characterisation of a single-centre case series. Acta Neurochir (Wien) 165:1615–1633. https://doi.org/10.1016/j.neurad.2022.12.001
Chen CCL, Deshmukh S, Jessa S, Hadjadj D, Lisi V, Andrade AF, Faury D, Jawhar W, Dali R, Suzuki H, Pathania M, Dubois AD, Woodward F, Hébert E, Coutelier S, Karamchandani M, Albrecht J, Brandner S, De Jay S, Gayden N, Bajic T, Harutyunyan A, Marchione AS, Mikael DM, Juretic LG, Zeinieh N, Russo M, Maestro C, Bassenden N, Hauser AV, Virga P, Bognar J, Klekner L, Zapotocky A, Vicha M, Krskova A, Vanova L, Zamecnik K, Sumerauer J, Ekert D, Ziegler PG, Ellezam DS, Filbin B, Blanchette MG, Hansford M, Khuong-Quang JR, Berghuis DA, Weil AM, Garcia AG, Garzia BA, Mack L, Beroukhim SC, Ligon R, Taylor KL, Bandopadhayay MD, Kramm P, Pfister C, Korshunov SM, Sturm A, Jones D, Salomoni DTW, Kleinman P, Jabado CL N (2020) Histone H3.3G34-Mutant Interneuron progenitors co-opt PDGFRA for Gliomagenesis. Cell 183:1617–1633e1622. https://doi.org/10.1016/j.cell.2020.11.012
Korshunov A, Capper D, Reuss D, Schrimpf D, Ryzhova M, Hovestadt V, Sturm D, Meyer J, Jones C, Zheludkova O, Kumirova E, Golanov A, Kool M, Schüller U, Mittelbronn M, Hasselblatt M, Schittenhelm J, Reifenberger G, Herold-Mende C, Lichter P, von Deimling A, Pfister SM, Jones DT (2016) Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol 131:137–146. https://doi.org/10.1007/s00401-015-1493-1
Barsouk A, Baldassari MP, Khanna O, Andrews CE, Ye DY, Velagapudi L, Al Saiegh F, Hafazalla K, Cunningham E, Patel H, Malkani K, Fitchett EM, Farrell CJ, Judy KD (2021) Glioblastoma with deep supratentorial extension is associated with a worse overall survival. J Clin Neurosci 93:82–87. https://doi.org/10.1016/j.jocn.2021.08.025
Dayani F, Young JS, Bonte A, Chang EF, Theodosopoulos P, McDermott MW, Berger MS, Aghi MK (2018) Safety and outcomes of resection of butterfly glioblastoma. Neurosurg Focus 44:E4. https://doi.org/10.3171/2018.3.FOCUS1857
Fujii K, Hirano S, Kurozumi K, Date I (2023) A case of high-Grade Glioma in an eloquent area treated with Awake Craniotomy in an 85-year-old patient. Acta Med Okayama 77(3):335–340. https://doi.org/10.18926/AMO/65504
Acknowledgements
We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study was supported by grants-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 22K16687 to YO and No. 23K08569 to JI).
Open Access funding provided by Okayama University.
Author information
Authors and Affiliations
Contributions
Conception and Design: YO Acquisition of Data: YK, YI Analysis and Interpretation of Data: YK, YO Drafting the Article: YK, YO Critically Revising the Article: YO, RM, YI, FH, JI, KF, NY Reviewed submitted version of manuscript: KW, SK, KK Approved the final version of the manuscript on behalf of all authors (corresponding author only): YO Statistical analysis: YK, YT Administrative / technical / material support: KW, JI, KF Study supervision: ID.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kegoya, Y., Otani, Y., Inoue, Y. et al. Midline invasion predicts poor prognosis in diffuse hemispheric glioma, H3 G34-mutant: an individual participant data review. J Neurooncol 167, 201–210 (2024). https://doi.org/10.1007/s11060-024-04587-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04587-5