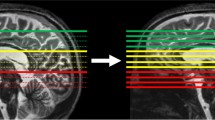
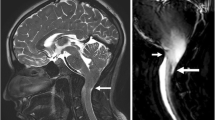
Abstract
Purpose
To review the evolution of cross-sectional imaging in pediatric neuroradiology from early developments to current advancements and future directions.
Methods
Information was obtained through a PubMed literature search as well as referenced online resources and personal experience from radiologists currently practicing pediatric neuroimaging and those who experienced the era of nascent cross-sectional imaging.
Results
The advent of computed tomography (CT) and magnetic resonance imaging (MRI) in the 1970s and 1980s brought about a revolutionary shift in the field of medical imaging, neurosurgical and neurological diagnosis. These cross-sectional imaging techniques ushered in a new era by enabling the visualization of soft tissue structures within the brain and spine. Advancements in these imaging modalities have continued at a remarkable pace, now providing not only high high-resolution and 3-dimensional anatomical imaging, but also functional assessment. With each stride forward, CT and MRI have provided clinicians with invaluable insights, improving the accuracy and precision of diagnoses, facilitating the identification of optimal surgical targets, and guiding the selection of appropriate treatment strategies.
Conclusion
This article traces the origins and early developments of CT and MRI, chronicling their journey from pioneering technologies to their current indispensable status in clinical applications and exciting possibilities that lie ahead in the realm of medical imaging and neurologic diagnosis.



Similar content being viewed by others
Change history
22 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00381-023-06132-7
References
Alexander RE, Gunderman RB (2010) EMI and the first CT scanner. J Am Coll Radiol 7(10):778–781. https://doi.org/10.1016/j.jacr.2010.06.003
Goodman LR (2010) The Beatles, the Nobel Prize, and CT scanning of the chest. Thorac Surg Clin 20:(1)1–7. https://doi.org/10.1016/j.thorsurg.2009.12.001
Schulz RA, Stein JA, Pelc NJ (2021) How CT happened: the early development of medical computed tomography. J Med Imaging (Bellingham) 8(5):052110. https://doi.org/10.1117/1.JMI.8.5.052110
Beckmann EC (2006) CT scanning the early days. Br J Radiol 79(937):5–8. https://doi.org/10.1259/bjr/29444122
Ambrose J (1973) Computerized transverse axial scanning (tomography). 2. Clinical application. Br J Radiol 46(552):1023–47. https://doi.org/10.1259/0007-1285-46-552-1023
Hounsfield GN (1973) Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol 46(552):1016–1022. https://doi.org/10.1259/0007-1285-46-552-1016
Petrik V, Apok V, Britton JA, Bell BA, Papadopoulos MC (2006) Godfrey Hounsfield and the dawn of computed tomography. Neurosurgery 58(4):780–7; discussion 780–7. https://doi.org/10.1227/01.NEU.0000204309.91666.06
Rubin GD (2014) Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 273(2 Suppl):S45-74. https://doi.org/10.1148/radiol.14141356
Brenner DJ, Hall EJ (2007) Computed tomography–an increasing source of radiation exposure. N Engl J Med 357(22):2277–2284. https://doi.org/10.1056/NEJMra072149
Seeram E (2018) Computed tomography: a technical review. Radiol Technol 89(3):279CT-302CT. Available: https://www.ncbi.nlm.nih.gov/pubmed/29298954
Lell MM, Wildberger JE, Alkadhi H, Damilakis J, Kachelriess M (2015) Evolution in computed tomography: the Battle for speed and dose. Invest Radiol 50(9):629–644. https://doi.org/10.1097/RLI.0000000000000172
Goldman LW (2008) Principles of CT: multislice CT. J Nucl Med Technol 36(2):57–68; quiz 75–6. https://doi.org/10.2967/jnmt.107.044826
Forghani R, De Man B, Gupta R (2017) Dual-energy computed tomography: physical principles, approaches to scanning, usage, and implementation: part 2. Neuroimaging Clin N Am 27(3):385–400. https://doi.org/10.1016/j.nic.2017.03.003
Postma AA, Das M, Stadler AA, Wildberger JE (2015) Dual-energy CT: what the neuroradiologist should know. Curr Radiol Rep 3(5):16. https://doi.org/10.1007/s40134-015-0097-9
Tran NA, Sodickson AD, Gupta R, Potter CA (2022) Clinical applications of dual-energy computed tomography in neuroradiology. Semin Ultrasound CT MR 43(4):280–292. https://doi.org/10.1053/j.sult.2022.03.003
Hsieh SS, Leng S, Rajendran K, Tao S, McCollough CH (2021) Photon counting CT: clinical applications and future developments. IEEE Trans Radiat Plasma Med Sci 5(4):441–452. https://doi.org/10.1109/trpms.2020.3020212
Pourmorteza A et al (2017) Photon-counting CT of the brain: in vivo human results and image-quality assessment. AJNR Am J Neuroradiol 38(12):2257–2263. https://doi.org/10.3174/ajnr.A5402
Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, Fleischmann D (2018) Photon-counting CT: technical principles and clinical prospects. Radiology 289(2):293–312. https://doi.org/10.1148/radiol.2018172656
Dillon WP (2021) 50th anniversary of computed tomography: past and future applications in clinical neuroscience. J Med Imaging (Bellingham) 8(5):052112. https://doi.org/10.1117/1.JMI.8.5.052112
Wang LL, Leach JL, Breneman JC, McPherson CM, Gaskill-Shipley MF (2014) Critical role of imaging in the neurosurgical and radiotherapeutic management of brain tumors. Radiographics 34(3):702–21. https://doi.org/10.1148/rg.343130156
Berrington de Gonzalez A et al (2009) Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169(22):2071–2077. https://doi.org/10.1001/archinternmed.2009.440
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176(2):289–296. https://doi.org/10.2214/ajr.176.2.1760289
Huang WY et al (2014) Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer 110(9):2354–2360. https://doi.org/10.1038/bjc.2014.103
Mathews JD et al (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians, 346:f2360. https://doi.org/10.1136/bmj.f2360
Miglioretti DL et al (2013) The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 167(8):700–707. https://doi.org/10.1001/jamapediatrics.2013.311
Pearce MS et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380(9840):499–505. https://doi.org/10.1016/S0140-6736(12)60815-0
National Research Council (1998) In Health risks from exposure to low levels of ionizing radiation: BEIR VII, phase I, letter report (1998). Washington (DC)
Kodama K, Ozasa K, Okubo T (2012) Radiation and cancer risk in atomic-bomb survivors. J Radiol Prot 32(1):N51–N54. https://doi.org/10.1088/0952-4746/32/1/N51
Wakeford R (2013) The risk of childhood leukaemia following exposure to ionising radiation–a review. J Radiol Prot 33(1):1–25. https://doi.org/10.1088/0952-4746/33/1/1
Nagayama Y et al (2018) Radiation dose reduction at pediatric CT: use of low tube voltage and iterative reconstruction. Radiographics 38:(5):1421–1440. https://doi.org/10.1148/rg.2018180041
Strauss KJ et al (2010) Image gently: ten steps you can take to optimize image quality and lower CT dose for pediatric patients. AJR Am J Roentgenol 194(4):868–873. https://doi.org/10.2214/AJR.09.4091
Schild HH (1994) MRI made easy: (...well almost) Berlex Laboratories, p. 105
Pooley RA (2005) AAPM/RSNA physics tutorial for residents: fundamental physics of MR imaging (in eng). Radiographics 25(4):1087–99. https://doi.org/10.1148/rg.254055027
Roguin A (2004) Nikola Tesla: the man behind the magnetic field unit. J Magn Reson Imaging 19(3):369–374. https://doi.org/10.1002/jmri.20002
Rabi II, Zacharias JR, Millman S, Kusch P (1938) A new method of measuring nuclear magnetic moment. Phys Rev 53(4):318–318. https://doi.org/10.1103/PhysRev.53.318
Bloch F (1946) Nuclear induction. Phys Rev 70(7–8):460–474. https://doi.org/10.1103/PhysRev.70.460
Purcell EM, Torrey HC, Pound RV (1946) Resonance absorption by nuclear magnetic moments in a solid. Phys Rev 69(1–2):37–38. https://doi.org/10.1103/PhysRev.69.37
Gorter LFJBCJ (1942) Negative result of an attempt to observe nuclear magnetic resonance in solids. Phys Rev 9(6):591–596
Damadian R (1971) Tumor detection by nuclear magnetic resonance (in eng). Science 171(3976):1151–1153. https://doi.org/10.1126/science.171.3976.1151
Lauterbur PC (1989) Image formation by induced local interactions. Examples employing nuclear magnetic resonance 1973 (in eng). Clin Orthop Relat Res 244:3–6
Garroway AN, Grannell PK, Mansfield P (1974) Image formation in NMR by a selective irradiative process. J Phys C: Solid State Phys 7(24):L457
Kumar A, Welti D, Ernst RR (2011) NMR Fourier zeugmatography. 1975 (in eng). J Magn Reson 213(2):495–509. https://doi.org/10.1016/j.jmr.2011.09.019
Clow H, Young IR (1973) Britain’s brains produce first NMR scans. New Scientist 242:190–191
Bilaniuk LT et al (1984) “Cerebral magnetic resonance: comparison of high and low field strength imaging,” (in eng). Radiology 153(2):409–414. https://doi.org/10.1148/radiology.153.2.6541355
Ai T et al (2012) “A historical overview of magnetic resonance imaging, focusing on technological innovations,” (in eng). Invest Radiol 47(12):725–741. https://doi.org/10.1097/RLI.0b013e318272d29f
Zimmerman RA (1999) “Past, present and future of pediatric neuroradiology,” (in eng). Childs Nerv Syst 15(11–12):624–634. https://doi.org/10.1007/s003810050451
Wesbey GE, Moseley ME, Ehman RL (1984) Translational molecular self-diffusion in magnetic resonance imaging. II. Measurement of the self-diffusion coefficient (in eng). Invest Radiol 19(6):491–8. https://doi.org/10.1097/00004424-198411000-00005
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) “MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders,” (in eng). Radiology 161(2):401–407. https://doi.org/10.1148/radiology.161.2.3763909
Weinmann HJ, Brasch RC, Press WR, Wesbey GE (1984) “Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent,” (in eng). AJR Am J Roentgenol 142(3):619–624. https://doi.org/10.2214/ajr.142.3.619
Russell EJ et al (1987) “Multiple cerebral metastases: detectability with Gd-DTPA-enhanced MR imaging,” (in eng). Radiology 165(3):609–617. https://doi.org/10.1148/radiology.165.3.3317495
Runge VM (2008) Notes on “Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent.” AJR Am J Roentgenol 190(6):1433–1434. https://doi.org/10.2214/AJR.07.3549
Marckmann P et al (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17(9):2359–2362. https://doi.org/10.1681/ASN.2006060601
FDA (2018) FDA Drug Safety Communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction | FDA. https://www.fda.gov/Drugs/DrugSafety/ucm223966.htm. Accessed May 2023
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) “High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material,” (in eng). Radiology 270(3):834–841. https://doi.org/10.1148/radiol.13131669
McDonald JS et al (2017) Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr 171(7):705–707. https://doi.org/10.1001/jamapediatrics.2017.0264
McDonald RJ et al (2015) “Intracranial gadolinium deposition after contrast-enhanced MR imaging,” (in eng). Radiology 275(3):772–782. https://doi.org/10.1148/radiol.15150025
FDA (2022) FDA Drug Safety Podcast: FDA warns that gadolinium-based contrast agents (GBCAs are retained in the body; requires new class warnings. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body-requires. Accessed May 2023
Douek P, Turner R, Pekar J, Patronas N, Le Bihan D (1991) MR color mapping of myelin fiber orientation (in eng). J Comput Assist Tomogr 15:(6):923–9. https://doi.org/10.1097/00004728-199111000-00003
Kwong KK et al (1992) “Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation,” (in eng). Proc Natl Acad Sci USA 89(12):5675–5679. https://doi.org/10.1073/pnas.89.12.5675
Laub GA, Kaiser WA (1988) MR angiography with gradient motion refocusing (in eng). J Comput Assist Tomogr 12(3):377–82. https://doi.org/10.1097/00004728-198805010-00002
Marchal G et al (1990) Intracranial vascular lesions: optimization and clinical evaluation of three-dimensional time-of-flight MR angiography (in eng). Radiology 175(2):443–448. https://doi.org/10.1148/radiology.175.2.2326471
Bottomley PA, Edelstein WA, Foster TH, Adams WA (1985) In vivo solvent-suppressed localized hydrogen nuclear magnetic resonance spectroscopy: a window to metabolism? (in eng). Proc Natl Acad Sci USA 82(7):2148–2152. https://doi.org/10.1073/pnas.82.7.2148
Jahng GH, Li KL, Ostergaard L, Calamante F (2014) Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol 15(5):554–77. https://doi.org/10.3348/kjr.2014.15.5.554
Geubert (2022) Guerbet announces U.S. Food and Drug Administration (FDA) approval of Elucirem (Gadopiclenol). https://www.guerbet.com/news/guerbet-announces-u-s-food-and-drug-administration-fda-approval-of-elucirem-gadopiclenol. Accessed May 2023
Funke SKI et al (2022) Long-term gadolinium retention in the healthy rat brain: comparison between gadopiclenol, gadobutrol, and gadodiamide. Radiology 305(1):179–189. https://doi.org/10.1148/radiol.212600
Robic C et al (2019) Physicochemical and pharmacokinetic profiles of gadopiclenol: a new macrocyclic gadolinium chelate with high T1 relaxivity. Invest Radiol 54(8):475–484. https://doi.org/10.1097/RLI.0000000000000563
Rinck PA (2023) An excursion into the history of magnetic resonance imaging, 13 ed. Norderstadt, Germany: The Round Table Foundation
Smith-Bindman R et al (2019) Trends in use of medical imaging in US health care systems and in Ontario, Canada, 2000–2016. JAMA 322(9):843–856. https://doi.org/10.1001/jama.2019.11456
Brownell GL (1999) A history of positron imaging. Res Lab. Massachusetts General Hospital, MIT
Jones T, Townsend D (2017) History and future technical innovation in positron emission tomography. J Med Imaging (Bellingham) 4(1):011013. https://doi.org/10.1117/1.JMI.4.1.011013
Townsend DW (2008) Combined positron emission tomography-computed tomography: the historical perspective. Semin Ultrasound CT MR 29(4):232–235. https://doi.org/10.1053/j.sult.2008.05.006
Muzic RF Jr, DiFilippo FP (2014) Positron emission tomography-magnetic resonance imaging: technical review. Semin Roentgenol 49(3):242–254. https://doi.org/10.1053/j.ro.2014.10.001
Davis KM, Ryan JL, Aaron VD, Sims JB (2020) PET and SPECT Imaging of the brain: history, technical considerations, applications, and radiotracers (in eng). Semin Ultrasound CT MR 41(6):521–529. https://doi.org/10.1053/j.sult.2020.08.006
Fois A et al (1995) “EEG, PET, SPET and MRI in intractable childhood epilepsies: possible surgical correlations,” (in eng). Childs Nerv Syst 11(12):672–678. https://doi.org/10.1007/bf00262229
Frost R et al (2019) Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn Reson Med 82(1):126–144. https://doi.org/10.1002/mrm.27705
Hori M, Hagiwara A, Goto M, Wada A, Aoki S (2021) Low-field magnetic resonance imaging: its history and renaissance. Invest Radiol 56(11):669–679. https://doi.org/10.1097/RLI.0000000000000810
Knopp LAE (2021) Clinical use cases for the Hyperfine Swoop Portable MR imaging system
Martin D, Tong E, Kelly B, Yeom K, Yedavalli V (2021) Current perspectives of artificial intelligence in pediatric neuroradiology: an overview. Front Radiol 1. https://doi.org/10.3389/fradi.2021.713681
Duong MT, Rauschecker AM, Mohan S (2020) Diverse applications of artificial intelligence in neuroradiology. Neuroimaging Clin N Am 30(4):505–516. https://doi.org/10.1016/j.nic.2020.07.003
Koetzier LR et al (2023) Deep learning image reconstruction for CT: technical principles and clinical prospects. Radiology 306(3):e221257. https://doi.org/10.1148/radiol.221257
Lin DJ, Johnson PM, Knoll F, Lui YW (2021) Artificial intelligence for MR image Reconstruction: an overview for clinicians. J Magn Reson Imaging 53(4):1015–1028. https://doi.org/10.1002/jmri.27078
Lui YW et al (2020) Artificial intelligence in neuroradiology: current status and future directions. AJNR Am J Neuroradiol 41(8):E52–E59. https://doi.org/10.3174/ajnr.A6681
Acknowledgements
We would like to sincerely thank Dr. Eric J. Russell, Professor of Radiology at Feinberg School of Medicine, Northwestern Memorial Hospital, for his personal tales of the early times of CT and MRI and his generous lending of the images used in Fig. 2.
Author information
Authors and Affiliations
Contributions
Maura Ryan authored portions of the main manuscript (predominantly MRI), prepared Figures 2 and 3, edited manuscript, performed research and organization of the manuscript. Alok Jaju authored portions of main manuscript contributor (predominantly CT), prepared Figure 1, edited manuscript, performed research and organization of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the caption to Fig. 2: a) an “l” was omitted from the name of the physician contributing the image, b) “leptomeningeal metatstasis” should be “mycotic aneurysms”.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ryan, M.E., Jaju, A. Revolutionizing pediatric neuroimaging: the era of CT, MRI, and beyond. Childs Nerv Syst 39, 2583–2592 (2023). https://doi.org/10.1007/s00381-023-06041-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06041-9