Abstract
Background
Cerebral ventricular shunt infections caused by methicillin-resistant Staphylococcus aureus (MRSA), especially strains with elevated minimum inhibitory concentration (MIC) values, have a poor prognosis. Monitoring serum vancomycin (VCM) levels with therapeutic drug monitoring and maintaining high VCM concentrations in the cerebrospinal fluid (CSF) are critical to treatment success. However, there have been a few reports about the CSF penetration and the pharmacokinetics of VCM in children.
Case presentation
Here, we report the case of a pediatric patient with cysto-peritoneal shunt-related meningitis caused by MRSA with an MIC of 2 μg/mL. The adequate VCM concentration was maintained by monitoring the VCM concentration in the CSF via the external ventricular drain, and frequent blood taking was avoided. VCM showed a good CSF penetration in our patient, and she was discharged without complications.
Discussion
Therapeutic drug monitoring of VCM concentration in the CSF may result in successful treatment even if MRSA shows a higher MIC. Therapeutic drug monitoring of VCM concentration in the CSF may also reduce the side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Post-neurosurgical central nervous system (CNS) infections can occur in 1%–8.6% of cases and can be treated, but antimicrobial-resistant pathogens are a growing concern and limit treatment options [1,2,3,4]. Therapeutic drug monitoring (TDM) is recommended to ensure appropriate antimicrobial therapy for antimicrobial agents with a narrow range of efficacy and toxicity. The drug concentration is measured, and the optimal dosage and administration method are established based on pharmacokinetics/pharmacodynamics theory [5].
Vancomycin (VCM) has a time-dependent activity with limited penetration in the cerebrospinal fluid (CSF) because of its hydrophilicity and large molecular size [5]. Therefore, a high serum concentration is needed to achieve an appropriate CSF concentration. However, the penetration of VCM is variable and unpredictable, depending on patient factors [6, 7].
The recent guidelines recommend VCM for treating CNS infections caused by methicillin-resistant Staphylococcus aureus (MRSA). However, these recommendations are primarily based on data from adult populations. They also propose a second-line drug if the strain’s minimum inhibitory concentration (MIC) value is 2 μg/mL [1].
Here, we report the case of a pediatric patient with cysto-peritoneal shunt-related infection caused by MRSA with MIC value of 2 μg/mL. We successfully treated with VCM by monitoring both CSF and serum concentration levels of VCM.
Case presentation
A 2-year-old girl with congenital intracranial cysts underwent endoscope-assisted fenestration and shunt valve replacement surgery against cysto-peritoneal shunt dysfunction. The pregnancy was uneventful and the child was born normally at 3,214 g, 49.0 cm height, and 43.1 cm head circumference. She had congenital intracranial cysts and a cerebral malformation was detected by pathological examination at 8 days of age. A cysto-peritoneal shunt was inserted 1 month after birth.
Four days after the operation (day 1), she developed a fever and irritability. On physical examination, she was febrile, had a Glasgow Coma Scale of E3V5M6, and showed nuchal rigidity. The patient’s laboratory data were white blood cell count, 13.7 × 109/L and C-reactive protein, 19.4 mg/dL. CSF examination showed a cell count of 236 /μL; total protein, 10.6 g/L; and glucose, 2.6 mmol/L. Gram staining of the CSF detected gram-positive cluster microorganisms. We initiated intravenous VCM. MRSA was isolated from the CSF on day 2; the MIC of VCM was 2 μg/mL (Table 1). The cysto-peritoneal shunt was removed, and an external ventricular drain (EVD) was placed on the same day. A shunt culture was also positive for MRSA.
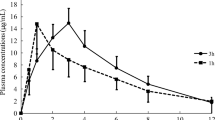
The VCM levels in the serum and CSF were measured during treatment (Table 2). The dosage of VCM was adjusted to achieve both a CSF trough level of at least 2.0 mg/L and a serum trough level of less than 20 mg/L, resulting between 70 − 100 mg/kg/d. The median serum trough level was 13.7 mg/L (interquartile range [IQR]: 10.7 − 15.2 mg/L), and the median CSF trough level was 5.3 mg/L (IQR: 4.1 − 5.9 mg/L), and the median CSF/serum concentration ratio was 0.29 mg/L (IQR: 0.28 − 0.42 mg/L). Her serum creatinine levels showed a normal range during treatment.
On day 3, her symptoms improved. CSF culture was no MRSA growth shown on day 6. On day16, brain magnetic resonance imaging was performed, and no findings of abscess formation showed. Antibiotic treatment was ended on day 29, and the patient was discharged without any complications on day 42.
Discussion
We successfully treated cysto-peritoneal shunt-related infection caused by MRSA with MIC of VCM of 2 μg/mL by monitoring both CSF and serum concentration levels of VCM. The penetration rate of VCM from the blood to CSF was shown to be sufficiently high.
The target concentration for CNS infection caused by MRSA with MIC of VCM of 2 μg/mL could not be achieved based on the in vitro data [6]. However, the guideline states that VCM can be continued if the patients improve clinically because one point of MIC difference can occur by laboratory error and the MIC result varies based on the method used [1, 8,9,10,11].
There has been no clear evidence of the safety and efficacy levels of VCM concentration in CSF. However, based on the data from intraventricular administration, the CSF trough levels > 10 times the MIC have not been associated with severe or irreversible adverse events [1]. Concerning indicators of efficacy, the CSF trough concentration above the MIC has been suggested in pediatric patients [8].
The penetration rate has been reported in the range of 0 − 68% in children (Table 3) [12,13,14,15]. A higher penetration rate by opening of the tight junctions of the blood–brain-barrier cells, delayed drug removal by a decrease of the CSF bulk flow, and inhibited activity by efflux pump of antibiotics have been occurred during the acute phase of bacterial meningitis [6, 7, 15, 16]. Otherwise, intense inflammation was not regularly present in cerebral ventricular shunt-related infections [7, 17]. However, there have been some reports that the patients with cerebral ventricular shunt- or EVD-related infection showed relatively higher levels of antibiotics concentration in the CSF than those without these devices because of the disruption of the blood-CSF barrier [14, 18,19,20].
Several studies have reported that intraventricular use of VCM may improve treatment outcomes without severe side effects in adult patients [21,22,23]. However, arecent systematic review noted insufficient evidence in pediatric patients, and intraventricular antimicrobial therapy is considered when clinical improvement is poor with intravenous administration alone [24].
In our patient, although the strain isolated from the CSF showed MIC of VCM of 2 μg/mL, successful treatment with intravenous VCM was achieved by monitoring the concentration in both the serum and CSF. The penetration rate was sufficiently high and the CSF trough levels were above the MIC during treatment.
High serum VCM concentration can cause complications such as nephrotoxicity, ototoxicity, and vasculitis [4]. We could avoid unnecessary dose increases by monitoring the CSF concentration, which may lead to excellent tolerance and no clinically significant adverse events.
In conclusion, monitoring the VCM concentration in the CSF and its serum concentration as indicators may help make decisions about the optimal dosage, changing second-line drugs, and reducing the frequency of side effects.
Availability of data and materials
The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- EVD:
-
External ventricular drain
- IQR:
-
Interquartile range
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MIC:
-
Minimal inhibitory concentration
- TDM:
-
Therapeutic drug monitoring
- VCM:
-
Vancomycin
References
Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Schld WM et al (2017) 2017 Infectious Disease Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis 64:e34–e65
Karvouniaris M, Brotis A, Tsiakos K, Palli E, Koulenti D (2022) Current perspectives on the diagnosis and management of healthcare-associated ventriculitis and meningitis. Infect Drug Resist 15:697–721
McClelland S, Hall WA (2007) Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis 45:55–59
Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B et al (2009) Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. J Neurosurg Pediatr 4:156–165
Arda B, Yamazhan T, Sipahi OR, Islekel S, Buke C, Ulusoy S (2005) Meningitis due to methicillin-resistant Staphylococcus aureus (MRSA): review of 10 cases. Int J Antimicrob Agents 25:414–418
Rybak MJ (2006) The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42:S35–S39
Beach JE, Perrott J, Turgeon RD, Ensom MHH (2017) Penetration of vancomycin into the cerebrospinal fluid: a systematic review. Clin Pharmacokinet 56:1479–1490
Nau R, Sorgel F, Eiffert H (2010) Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883
Kumta N, Roberts JA, Lipman J, Cotta MO (2018) Antibiotic distribution into cerebrospinal fluid: can dosing safely account for drug and disease factors in the treatment of ventriculostomy-associated infections? Clin Pharmacokinet 57:439–454
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ et al (2011) Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18-55
van Hal SJ, Lodise TP, Paterson DL (2012) The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 54:755–771
Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME (2014) Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 312:1552–1564
Baxi SM, Clemenzi-Allen A, Gahbauer A, Gahbauer A, Deck D, Imp B et al (2016) Vancomycin MIC does not predict 90-day mortality, readmission, or recurrence in a prospective cohort of adults with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 60:5276–5284
Jorgenson L, Reiter PD, Freeman JE, Winston KR, Fish D, McBride LA et al (2007) Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr Neurosurg 43:449–455
Klugman KP, Friedland IR, Bradley JS (1995) Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother 39:1988–1992
Reiter PD, Doron MW (1996) Vancomycin cerebrospinal fluid concentrations after intravenous administration in premature infants. J Perinatol 16:331–335
Autmizguine J, Moran C, Gonzalez D, Capparelli EV, Smith PB, Grant GA et al (2014) Vancomycin cerebrospinal fluid pharmacokinetics in children with cerebral ventricular shunt infections. Pediatr Infect Dis J 33:e270–e272
Scheld WM, Dacey RG, Winn HR, Welsh JE, Jane JA, Sande MA et al (1980) Cerebrospinal fluid outflow resistance in rabbits with experimental meningitis. Alterations with penicillin and methylprednisolone. J Clin Invest 66:243–253
Wang Q, Shi Z, Wang J, Shi G, Wang S, Zhou J (2008) Postoperatively administered vancomycin reaches therapeutic concentration in the cerebral spinal fluid of neurosurgical patients. Surg Neurol 69:126–129
Ichie T, Urano K, Suzuki D, Okada T, Kobayashi N, Hayashi H et al (2015) Influence of cerebrospinal fluid drainage on the pharmacokinetics of vancomycin in neurosurgical patients. Pharmazie 70:404–409
Fan-Havard P, Nahata MC, Bartkowski MH, Barson WJ, Kosnik EJ (1990) Pharmacokinetics and cerebrospinal fluid (CSF) concentrations of vancomycin in pediatric patients undergoing CSF shunt placement. Chemotherapy 36:103–108
Wilkie MD, Hanson MF, Statham PF, Brennan PM (2013) Infections of cerebrospinal fluid diversion devices in adults: the role of intraventricular antimicrobial therapy. J Infect 66:239–246
Ng K, Mabasa VH, Chow I, Ensom MH (2014) Systematic review of efficacy, pharmacokinetics, and administration of intraventricular vancomycin in adults. Neurocrit Care 20:158–171
Chen K, Wu Y, Wang Q et al (2015) The methodology and pharmacokinetics study of intraventricular administration of vancomycin in patients with intracranial infections after craniotomy. J Crit Care 30(218):e1–5
Author information
Authors and Affiliations
Contributions
Dr. SM conceptualized the study, collected data, analyzed and interpreted data, drafted the initial manuscript, and critically reviewed and revised the manuscript. Drs. JK, HK, and MK collected data, drafted the initial manuscript, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of the Kobe Children’s Hospital (no. R4-143) approved this study protocol.
Consent for publication
Informed consent was obtained from the parents of the patient.
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mizuno, S., Koyama, J., Kurosawa, H. et al. Treatment optimization by monitoring vancomycin concentration in the serum and cerebrospinal fluid in a child with cystoperitoneal shunt-related infection caused by methicillin-resistant Staphylococcus aureus: a case report and literature review. Childs Nerv Syst 39, 3307–3310 (2023). https://doi.org/10.1007/s00381-023-06004-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06004-0