Abstract
Pontine gliomas represent difficult to treat entity due to the location and heterogeneous biology varying from indolent low-grade gliomas to aggressive diffuse intrinsic pontine glioma (DIPG). Making the correct tumor diagnosis in the pontine location is thus critical. Here, we report a case study of a 14-month-old patient initially diagnosed as histone H3 wild-type DIPG. Due to the low age of the patient, the MRI appearance of DIPG, and anaplastic astrocytoma histology, intensive chemotherapy based on the HIT-SKK protocol with vinblastine maintenance chemotherapy was administered. Rapid clinical improvement and radiological regression of the tumor were observed with nearly complete remission with durable effect and excellent clinical condition more than 6.5 years after diagnosis. Based on this unexpected therapeutic outcome, genome-wide DNA methylation array was employed and the sample was classified into the methylation class “Low-grade glioma, MYB(L1) altered.” Additionally, RT-PCR revealed the presence of MYB::QKI fusion. Taken together, the histopathological classification, molecular-genetic and epigenetic features, clinical behavior, and pontine location have led us to reclassify the tumor as a pontine MYB-altered glioma. Our case demonstrates that more intensive chemotherapy can achieve long-term clinical effect in the treatment of MYB-altered pontine gliomas compared to previously used LGG-based regimens or radiotherapy. It also emphasizes the importance of a biopsy and a thorough molecular investigation of pontine lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric pontine gliomas represent the most challenging diagnosis in pediatric oncology due to the location and heterogeneous biology varying from indolent low-grade gliomas to aggressive diffuse intrinsic pontine glioma (DIPG) [1, 2]. DIPG is one of the deadliest tumors of childhood with a lack of curative treatment options. The majority of these tumors harbor mutations in histone H3 and are hence classified as diffuse midline gliomas H3 K27-altered. Only anecdotal cases with long-term survival have been reported so far. Making the correct tumor diagnosis in the pontine location is thus critical. Nevertheless, the majority of international institutions initiate palliative focal radiation therapy based on clinical symptoms and MRI characteristics without previous biopsy.
Here, we describe a unique case of a very young child with histone H3 wild-type DIPG that was later characterized by the presence of MYB::QKI fusion treated with radiation sparing approach.
Case presentation
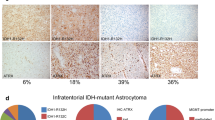
At the time of diagnosis in 2016, a 14-month-old previously healthy boy presented with a 2-month history of cranial nerve palsies (n. VI and n. VII in the left side) and progressive psychomotor regression. Brain MRI revealed a T2/FLAIR hyperintense, T1 hypointense, non-enhancing extensive intraaxial posterior fossa mass centered in the pons and causing hydrocephalus. Based on MRI and clinical characteristics, the diagnosis was consistent with DIPG; however, the age at presentation was rather atypical (Fig. 1A–C). A posterior fossa craniotomy with a tumor biopsy was performed. Histopathological examination revealed a moderately cellular tumor composed of medium-sized multipolar or elongated glial fibrillary acidic protein (GFAP)–positive astrocytes with projections creating a fibrillar background. A pathologist classified this tumor as anaplastic astrocytoma grade 3 as per the 3rd version of the WHO Classification of Central Nervous Tumors from 2007 (Fig. 2A–C) [3]. Direct sequencing of the tumor tissue sample was performed at the time of diagnosis using primers described elsewhere, and neither mutations exon 15 of BRAF gene nor histone 3 (H3F3A, HIST3B1) was present [4, 5]. Reverse transcription PCR (RT-PCR) did not reveal any variant of the KIAA1549::BRAF fusion [6].
Photomicrographs of the representative tumor sections. A Hematoxylin and eosin–stained slide shows moderately cellular glioma with mild cytological atypia and with no obvious growth pattern. B Immunohistochemical staining for CD34 is negative in tumor cells and positive in endothelial cells. It highlights the absence of perivascular growth pattern. C Proliferative activity of tumor cells shown by Ki-67 positivity is mildly increased (generally with relatively low positivity (up to 5%), but in several hotspots clearly increased (about 10%). D Schematic display of MYB::QKI fusion transcript
Due to the child’s age and better outcome of DIPG in young children (less than 3 years old) [7], intensive chemotherapy was initiated according to the German HIT-SKK-based regimen for infant high-grade glioma, consisting of 39 weeks of alternating cycles of vincristine plus cyclophosphamide, high-dose methotrexate, and carboplatin plus etoposide [8, 9]. Rapid clinical improvement followed by significant radiological partial tumor regression (> 50%) was achieved after 6 months (Fig. 3A, B). After completion of intensive chemotherapy, the patient continued with maintenance vinblastine monotherapy at a dose of 6 mg/m2 for a total duration of 70 weeks. Vinblastine was reduced to 5 mg/m2 due to hematological toxicity. At the end of the treatment, the MRI showed almost complete regression of the disease (Fig. 3C). Our patient is currently more than 6.5 years after the diagnosis with insignificant residual changes (T2-weighted images) in the pons (Fig. 3D). Clinically, he is in excellent condition without neurological deficits, living the life of a healthy child.
This favorable treatment response to chemotherapy alone and sustained durable remission were highly unlikely to occur in DIPG. Therefore, molecular analysis was expanded to evaluate true tumor biology and uncover underlying genetic aberrations. Genome-wide DNA methylation array was performed, and the sample was classified using the v12.5 version of the Heidelberg classifier into the methylation class “Low-grade glioma, MYB(L1) altered” with a calibrated score of 0.90 [10]. Consequently, we performed RT-PCR, which revealed the presence of MYB::QKI fusion (ex15::ex5) as displayed in Fig. 2D [11]. Taken together, the histopathological classification, molecular-genetic and epigenetic features, clinical behavior, and pontine location have led us to reclassify the tumor as a pontine MYB-altered glioma.
Discussion and conclusion
According to the fifth edition of the WHO Classification of Central Nervous System Tumors from 2021, pediatric MYB-altered gliomas are split into two main groups: “Diffuse astrocytoma, MYB- or MYBL1-altered” and “Angiocentric glioma” [12]. Both tumor types are predominantly localized in the cerebral cortex [13, 14] and are associated with epilepsy, and their resection usually has curative potential [15]. Genetically, they are characterized by the presence of the MYB alteration; angiocentric gliomas mostly by MYB::QKI gene fusion; in contrast, PCDHGA1, MMP16, and MAML are reported fusion partner genes of MYB/MYBL1 in diffuse astrocytomas [13].
However, only a few cases of angiocentric gliomas localized in the pons with a proven gene fusion MYB::QKI have been described in the literature so far [13, 16, 17]. Therapeutic LGG-based regimens used for these cases included vincristine plus carboplatin, bevacizumab, or mTOR inhibitor everolimus [16, 17]. The combined chemotherapy did not lead to a sufficient therapeutic response; only the addition of mTOR inhibitor in one patient provided stabilization of the disease [16]. Seven patients with MYB/MYBL1-altered brainstem gliomas were included in the single-center study from St. Jude Children’s Research Hospital. Radiation therapy was used in four out of five patients with treatment information available. All exhibited stable disease as the best response to the therapy [13]. We also reviewed the literature documenting cases of histopathologically confirmed angiocentric gliomas, but without any data on MYB/MYBL1 alteration available [17,18,19]. Reported use of a LGG-based regimen consisting of vincristine and carboplatin resulted in disease progression in treated patients (n = 2).
Despite the fact that MYB(L1)-altered gliomas represent group of indolent low-grade tumors with reported overall survival reaching 95% at 5 years [13], tumors within brainstem location frequently require therapy. In contrast to reported treatment outcomes, our patient achieved objective response and long-term remission of the disease with radiation sparing approach. Although this is an anecdotal experience based on a single patient, it suggests that an intensive chemotherapy regimen such as HIT-SKK chemotherapy could be more effective for MYB-altered diffuse astrocytomas or angiocentric gliomas localized in the pons.
Our experience further underscores the role of biopsy in patients with brain stem tumors with the distinctive MRI appearance of DIPG in all age groups, but especially in infants, where other entities outside DMG can be encountered. As exemplified by our case, diffuse brainstem gliomas with unknown drivers should be investigated for MYB alterations even if they do not histologically bear an angiocentric pattern.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Roux A, Pallud J, Saffroy R, Edjlali-Goujon M, Debily MA, Boddaert N, Sanson M, Puget S, Knafo S, Adam C et al (2020) High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro Oncol 22:1190–1202. https://doi.org/10.1093/neuonc/noaa024
Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M et al (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32:520–537. https://doi.org/10.1016/j.ccell.2017.08.017
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Agaimy A, Terracciano LM, Dirnhofer S, Tornillo L, Foerster A, Hartmann A, Bihl MP (2009) V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol 62:613–616. https://doi.org/10.1136/jcp.2009.064550
Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, Pages M, Taylor KR, Saulnier P, Lacroix L et al (2015) Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130:815–827. https://doi.org/10.1007/s00401-015-1478-0
Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP (2008) Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Can Res 68:8673–8677. https://doi.org/10.1158/0008-5472.CAN-08-2097
Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles L, Hawkins C et al (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol Off J Am Soc Clin Oncol 36:1963–1972. https://doi.org/10.1200/JCO.2017.75.9308
Kramm CM, Butenhoff S, Rausche U, Warmuth-Metz M, Kortmann RD, Pietsch T, Gnekow A, Jorch N, Janssen G, Berthold F et al (2011) Thalamic high-grade gliomas in children: a distinct clinical subset? Neuro Oncol 13:680–689. https://doi.org/10.1093/neuonc/nor045
Rutkowski S, Gerber NU, von Hoff K, Gnekow A, Bode U, Graf N, Berthold F, Henze G, Wolff JE, Warmuth-Metz M et al (2009) Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol 11:201–210. https://doi.org/10.1215/15228517-2008-084
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474. https://doi.org/10.1038/nature26000
Bandopadhayay P, Ramkissoon LA, Jain P, Bergthold G, Wala J, Zeid R, Schumacher SE, Urbanski L, O’Rourke R, Gibson WJ et al (2016) MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet 48:273–282. https://doi.org/10.1038/ng.3500
Figarella-Branger D, Appay R, Metais A, Tauziede-Espariat A, Colin C, Rousseau A, Varlet P (2022) The 2021 WHO Classification of Tumours of the Central Nervous System. Ann Pathol 42:367–382. https://doi.org/10.1016/j.annpat.2021.11.005
Chiang J, Harreld JH, Tinkle CL, Moreira DC, Li X, Acharya S, Qaddoumi I, Ellison DW (2019) A single-center study of the clinicopathologic correlates of gliomas with a MYB or MYBL1 alteration. Acta Neuropathol 138:1091–1092. https://doi.org/10.1007/s00401-019-02081-1
Ryall S, Zapotocky M, Fukuoka K, Nobre L, Guerreiro Stucklin A, Bennett J, Siddaway R, Li C, Pajovic S, Arnoldo A et al (2020) Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37:569–583. https://doi.org/10.1016/j.ccell.2020.03.011
Ampie L, Choy W, DiDomenico JD, Lamano JB, Williams CK, Kesavabhotla K, Mao Q, Bloch O (2016) Clinical attributes and surgical outcomes of angiocentric gliomas. J Clin Neurosci: Off J Nuerosurg Soc Australas 28:117–122. https://doi.org/10.1016/j.jocn.2015.11.015
D’Aronco L, Rouleau C, Gayden T, Crevier L, Decarie JC, Perreault S, Jabado N, Bandopadhayay P, Ligon KL, Ellezam B (2017) Brainstem angiocentric gliomas with MYB-QKI rearrangements. Acta Neuropathol 134:667–669. https://doi.org/10.1007/s00401-017-1763-1
Chan E, Bollen AW, Sirohi D, Van Ziffle J, Grenert JP, Kline CN, Tihan T, Perry A, Gupta N, Solomon DA (2017) Angiocentric glioma with MYB-QKI fusion located in the brainstem, rather than cerebral cortex. Acta Neuropathol 134:671–673. https://doi.org/10.1007/s00401-017-1759-x
Almubarak AO, Alahmari A, Al Hindi H, AlShail E (2020) Angiocentric glioma of brainstem. Neurosciences 25:416–420. https://doi.org/10.17712/nsj.2020.5.20200026
Weaver KJ, Crawford LM, Bennett JA, Rivera-Zengotita ML, Pincus DW (2017) Brainstem angiocentric glioma: report of 2 cases. J Neurosurg Pediatr 20:347–351. https://doi.org/10.3171/2017.5.PEDS16402
Funding
Open access publishing supported by the National Technical Library in Prague. This work was supported by the Charles University Grant Agency GAUK No. 204220 (KT, MZ); PRIMUS/19/MED/06 Charles University Grant Agency, Prague, Czech Republic (MZ, LK, AM, KT); MH CZ–DRO, University Hospital Motol, Prague, Czech Republic (00064203) (DS, AV, MZ); and the Project National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102)—Funded by the European Union—Next Generation EU. (MZ, KT, AV).
Author information
Authors and Affiliations
Contributions
Conceptualization: KT, MZ; methodology: KT, MZ, DS, AV, LK, MK; investigation: KT, MZ, DS, AV, LK, MK, VB; writing—original draft: KT, MZ; writing—review and editing: MZ, DS, KT; funding acquisition: MZ, KT, DS, AV, LK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for the study herein reported was provided by Institutional Ethics Committee of the Second Faculty of Medicine Charles University in Prague (ethics committee approval 17.6.2020).
Consent for publication
The authors of this publication declare that they have obtained the informed consent of the patient’s legal representatives for the publication of their anonymized data for this case report.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trkova, K., Sumerauer, D., Krskova, L. et al. DIPG-like MYB-altered diffuse astrocytoma with durable response to intensive chemotherapy. Childs Nerv Syst 39, 2509–2513 (2023). https://doi.org/10.1007/s00381-023-05976-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-05976-3