Abstract
Background
Hydrocephalus is a challenge for paediatric neurosurgeons. When the abdominal cavity and heart fail as diversion sites for cerebrospinal fluid (CSF), many of the otherwise used alternative diversion sites are not feasible due to the smaller physical body size of children and infants. Using the urinary system as a site of diversion has been described in adults primarily.
Objective
To describe a minimally invasive procedure to percutaneously access the ureter for placement of a distal catheter in the treatment of paediatric hydrocephalus.
Methods
A percutaneous ultrasound-assisted technique was used to access the renal pelvis for catheter placement into the distal ureter.
Results
Fifteen months after the surgery, the child has a stable neurological condition and adequately managed hydrocephalus.
Conclusion
The urinary tract should be considered a viable option for CSF diversion in complex paediatric hydrocephalus. A multidisciplinary approach consisting of interventional radiologists, urologists and neurosurgeons should be involved in the evaluation of potential candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex hydrocephalus is a challenge among prematurely born children with posthemorrhagic hydrocephalus. More than 80% of shunt patients require revision and more than half of the hydrocephalic patients are subjected to more than four surgeries [1]. The standard method of diverting cerebrospinal fluid (CSF) into the abdominal cavity is not always feasible. Sometimes, peritoneal resorptive insufficiency arises making other sites of diversion necessary. Current literature describes up to 36 sites of diversion varying from the mastoid bone to the pleura and the fallopian tubes [1]. Finding a site of diversion in a paediatric patient is challenging due to the small size of the patient and often high drainage volumes. The ventriculo-ureteral (VU) shunt is rarely used by the neurosurgical community and not many cases have been reported. Most of the reported cases are adult individuals and a percutaneous technique has rarely been used. In this paper, we describe a complex paediatric case that was successfully managed by the use of a percutaneously inserted VU shunt.
Case description
The patient is a premature boy, born in gestation week 26 + 2 with a birth weight of 1068 g. He was treated in neonatal intensive care and suffered from both necrotizing enterocolitis and grade IV intraventricular haemorrhage and posthemorrhagic hydrocephalus. His abdominal condition required two laparotomies with intestinal resections, while his posthemorrhagic condition included 32 shunt procedures/revisions (Table 1). He initially underwent implantation of a ventriculoperitoneal (VP) shunt, which due to infection and peritoneal malabsorption was replaced with a ventriculoatrial (VA) shunt. Due to atrial catheter malfunction, a VP shunt was reimplanted. However, also this VP shunt had to be replaced with another VA shunt, secondary to problems with malabsorption and the development of a CSF cyst that caused right-sided hydronephrosis. Unfortunately, extensive central venous thrombosis with subsequent vena cava syndrome eventually required the removal of the second VA shunt. A pleural shunt was now being considered, but due to the large drainage volumes, this was abandoned. The urinary bladder was also considered, but due to episodes of documented non-symptomatic bacteriuria during the past year, this was believed to be a risky endeavour. Finally, a VU shunt remained a viable option, and while acknowledging the risks of urinary tract infections as well as the risk of the shunt becoming a nidus for the formation of urinary calculi, the VU shunt was deemed the best option for this patient.
The parents have consented to the publication of the case.
Preoperative work-up
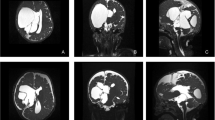
An MRI of the brain was performed to rule out hematoma, subdural collections, or new adhesions. Due to a left-sided posthemorrhagic ventricular dilatation, this side was chosen for the proximal catheter. CSF cultures were performed and found negative. Repeated urinary cultures showed no signs of bacteriuria. Persistent hydronephrosis was ruled out by ultrasound. Abdominal MRI showed that both kidneys were normal in size with adequate parenchymal thickness. The right ureter was found to be slightly wider than the left. A review of a previous CT scan showed a right-sided ureteric dilatation, sufficient to harbour the distal catheter. A voiding cystourethrography (VCUG) was performed to rule out vesicoureteral reflux while the bladder volume was estimated to be approximately 150 ml. A high-pressure bladder was considered unlikely, as there were no signs of bladder trabeculation or diverticulae on VCUG and both ultrasound and MRI showed a thin-walled bladder of normal size. Cystometry was not performed.
Surgical technique
The child was positioned semi-prone with his right side slightly elevated. A radiologist experienced in ultrasound-assisted percutaneous nephrostomies (MJ) and a paediatric urologist (JS) were present for the implantation of the distal catheter. The renal pelvis was reached with a micropuncture technique (0.9-mm needle and 0.018-in. guidewire) under ultrasound guidance and fluoroscopic control. Using the Seldinger technique and serial dilatation to 8 F, PTFE-coated stainless steel, 0.035-in. (0.89 mm) guidewire was placed via the ureter into the bladder (Fig. 1). After a small skin incision, the distal shunt catheter was then placed 3 cm cranially to the ureteric orifice and the final position was verified with fluoroscopy (Fig. 2). The shunt catheter was then tunnelled subcutaneously over the back, medially to the right scapula and connected to the shunt valve of a left-sided frontal ventricular catheter (inserted by neurosurgeons JB and US, Fig. 3).
On postoperative day 2, the child developed new neurological symptoms and a CT of the abdominal cavity showed that the distal catheter had migrated 2 cm distally, close to the ureteric orifice where the ureter typically is slightly narrower. There is also the possibility of the tubing being clogged by blood. The problem was resolved by shortening and flushing the distal catheter. The catheter was shortened by 2 cm (neurosurgeon EE). After this revision, the recovery was uneventful.
Outcome
Follow-up at 6 months included urinary cultures and a low-dose CT to rule out the development of hydronephrosis or urinary calculi, as well as any possible formation of calcifications on the distal catheter. The patient remains on a daily dose of oral prophylactic antibiotics (trimethoprim-sulfamethoxazole). At the 15-month follow-up, there have been no urinary tract or CNS infections, and no further episodes of shunt dysfunction. A new MRI of the brain was performed 1 year postoperatively and showed expected ventricular size and adequate location of the proximal catheter. No new abnormalities have emerged on radiological imaging, apart from those related to the pre-existing condition. There have not been any problems related to the maintenance of a normal electrolyte homeostasis and the patient has not required any form of fluid or electrolyte substitution. Neurological follow-up has consisted of out-patient appointments at the neurosurgical department, initially after 4 weeks and further every 6 months.
Discussion
Diversion of CSF into the genitourinary system was suggested as early as 1925 when Heile proposed an ureterodural anastomosis by suturing the renal pelvis into the lumbar dura [2]. In 1949, Matson described using a polyethene tube for drainage into the ureter [3]. The idea of utilizing the urinary tract for CSF diversion relies on rapid elimination through micturition rather than absorption [1, 4, 5].
The urinary bladder has long been used as an alternative diversion site in the treatment of hydrocephalus. Development of the VU shunt was initially described as a procedure including nephrectomy and was later developed into a ventriculo-pyelo-ureterostomy, a ventriculorenal shunt, where the distal end of the shunt catheter was placed in the pyelocaliceal area [1, 6]. Since the initial method of implanting a VU-shunt required removal of a kidney, this method never became widely adopted. Later, open surgical techniques sparing the kidney were described [7]. In the 1980s, Smith et al. described a technique of low ureteral transection for distal catheter placement, combined with re-implantation of the ureter, thus avoiding a nephrectomy [7]. The introduction of percutaneous nephrostomy has opened up new possibilities for minimally invasive access to the ureters as a diversion site [8].
Only a few cases of VU shunts have been reported in the literature and several case reports describe adult patients where the size of the ureters is more favourable for this procedure [6, 7, 9,10,11,12,13,14,15,16,17]. One of the few long-term follow-ups described good results in four patients, provided they had a low-pressure urinary bladder without urinary tract infections [16]. This study included a child, two teenagers, and a 29-year-old man, both of them having an open surgical approach to the ureter. The study describes a 5-year mean survival of the shunt, but all patients eventually needed a re-operation [16]. Complications such as shunt obstruction, infection (with and without associated urinary tract infection), migration or kinking of the tubing, and metabolic complications were described [16]. Many failures have been described to be due to calcification of the distal catheter, which is a known complication of ureteral stenting [9]. There is also the theoretical risk for retrograde reflux of urine into CSF spaces, although this has not been described in any cases [12]. A high-pressure bladder should be ruled out before considering a VU shunt. It also seems that patients with VU shunts might be prone to symptomatic electrolyte imbalance in situations of dehydration or gastroenteritis [3, 12]. Other potential long-term problems in addition to calcifications include biofilm formation on the shunt and erosion of the ureteral wall due to the catheter [12, 18]. Percutaneous insertion, with similar techniques as the one we used, has previously been described by Pillai et al. and Subramanian et al., where two adults with postinfectious and posttraumatic hydrocephalus were successfully treated [8, 12]. To the best of our knowledge, this is the first description of the percutaneous insertion of a VU shunt in a child.
Conclusion
We have demonstrated the successful use of a percutaneously inserted VU-shunt in a 4-year-old child. The ureters have a high capacity for distension and can accommodate shunts with a larger diameter than those typically recommended for ureteric stenting in children. VU-shunting can thus provide an alternative for salvage CSF drainage even in relatively small children. A multidisciplinary team consisting of neurosurgeons, urologists, and interventional radiologists is essential in evaluating suitable candidates.
Availability of data and materials
Not applicable.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- VU shunt:
-
Ventriculo-ureteral shunt
- VP shunt:
-
Ventriuloperitoneal shunt
- VCUG:
-
Voiding cystourethrography
- F:
-
French scale
References
Morosanu CO, Filip GA, Nicolae L, Florian IS (2020) From the heart to the bladder-particularities of ventricular shunt topography and the current status of cerebrospinal fluid diversion sites. Neurosurg Rev 43(3):847–860. https://doi.org/10.1007/s10143-018-1033-2
Heile B (1925) Anastomosis between ureter and spinal dura to drain congenital hydrocephalus. Zbl Chir 52:2229
Matson DD (1949) A new operation for the treatment of communicating hydrocephalus: report of a case secondary to generalized meningitis. J Neurosurg 6(3):238–247. https://doi.org/10.3171/jns.1949.6.3.0238
Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML (1996) Permeability properties of the intact mammalian bladder epithelium. Am J Physiol 271(4 Pt 2):F886–F894. https://doi.org/10.1152/ajprenal.1996.271.4.F886
Ames CD, Jane JA Jr, Jane JA Sr, Campbell FG, Howards SS (2001) A novel technique for ventriculovesical shunting of congenital hydrocephalus. J Urol 165(4):1169–1171
Behrendt H, Nau HE (1987) Ventriculo-renal shunt in the therapy of hydrocephalus. Urologe A 26(6):331–3. Ventrikulo-renaler Shunt zur Therapie des Hydrocephalus
Smith JA Jr, Lee RE, Middleton RG (1980) Ventriculoureteral shunt for hydrocephalus without nephrectomy. J Urol 123(2):224–226. https://doi.org/10.1016/s0022-5347(17)55868-1
Subramaniam V, Ganapathy S, Paruchuri S (2020) Ventriculo-ureteric shunts, the last resort in complicated shunt patients. Interdisciplinary Neurosurgery 22:100805. https://doi.org/10.1016/j.inat.2020.100805
Lescure V, Descazeaud A, Caire F, Salle H (2021) The ventriculo-ureteral shunt: an underused, valuable neurosurgical approach. Neurochirurgie 67(6):640–642. https://doi.org/10.1016/j.neuchi.2020.12.007
Attai K, Kursh E, Persky L, Nulsen F (1972) Bilateral ureteroileostomy in children with ventriculo-peritoneal shunt. J Urol 108(3):474–476. https://doi.org/10.1016/S0022-5347(17)60778-X
Maggi G, Ambrosio A, Profeta G (1974) Value of the ventriculo ureteral shunt after the failure of other shunts for hydrocephalus (case report). J Neurosurg Sci 18(1):12–15
Pillai A, Mathew G, Nachimuthu S, Kalavampara SV (2017) Ventriculo-ureteral shunt insertion using percutaneous nephrostomy: a novel minimally invasive option in a patient with chronic hydrocephalus complicated by multiple distal ventriculoperitoneal shunt failures. J Neurosurg JNS 127(2):255–259. https://doi.org/10.3171/2016.8.Jns16342
Pittman T, Steinhardt G, Weberf T (1992) Ventriculo-ureteral shunt without nephrectomy. Br J Neurosurg 6(3):261–263. https://doi.org/10.3109/02688699209002936
Sarkar HKA (2013) Ventriculo-ureteric shunt surgery: thou shalt not be forgotten of me! Neurol India 61:448–450
Hetet J-F, Hamel O, Rigaud J et al (2004) Ventriculo-ureteric shunt without associated nephrectomy for the treatment of hydrocephalus. Progres en Urologie: Journal de L'association Francaise D'urologie et de la Societe Francaise D'urologie 14(3):390–3. discussion 393
Irby PB 3rd, Wolf JS Jr, Schaeffer CS, Stoller ML (1993) Long-term follow-up of ventriculoureteral shunts for treatment of hydrocephalus. Urology 42(2):193–197. https://doi.org/10.1016/0090-4295(93)90646-r
Ohaegbulam C, Peters C, Goumnerova L (2004) Multiple successful revisions of a ventriculoureteral shunt without nephrectomy for the treatment of hydrocephalus: case report. Neurosurgery 55(4):E1027–E1031
Tunney MM, Keane PF, Jones DS, Gorman SP (1996) Comparative assessment of ureteral stent biomaterial encrustation. Biomaterials 17(15):1541–1546. https://doi.org/10.1016/0142-9612(96)89780-8
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Ulrika Sandvik, Jiri Bartek Jr, Erik Edström, Jakob Stenman, and Mattias Jönsson conducted the surgery. The manuscript writing was performed by Ulrika Sandvik, and revision and literature search were performed by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The parents have consented to the publication of the case and the case description including follow-up is approved by the local ethics board (2018/1873–31).
Consent for publication
The parents have consented to the publication.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sandvik, U., Bartek, J., Edström, E. et al. Percutaneously inserted ventriculo-ureteral shunt as a salvage treatment in paediatric hydrocephalus: a technical note. Childs Nerv Syst 39, 249–254 (2023). https://doi.org/10.1007/s00381-022-05673-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05673-7