Abstract
Purpose
The current article describes an 11-year-old male who has aplastic anemia with an extremely rare condition, that is, concomitant posterior fossa SDH and spinal SDH.
Methods
This is a case report and review of literature.
Case presentation
This case presents an 11-year-old male known to have aplastic anemia complained of neck and back pain, headache, and persistent vomiting for 3 days. He had no history of head or spine trauma at all. His parents are relatives “positive consanguinity,” and his sister suffers from aplastic anemia. Clinical examination revealed severe pallor at the time of presentation, with no neurologic or locomotor deficit and positive Kernig’s sign.
Conclusion
Patients with aplastic anemia or any bleeding disorder conditions should be investigated thoroughly if symptoms denoted a CNS pathology. Concomitant cranial and spinal SDH rarely occurs, and more studies are advocated to be structured to investigate the specific pathophysiology and etiologies of this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous posterior fossa and spinal subdural hematomas (SDH) are extremely uncommon pathologies along with poorly elucidated pathogenesis. In addition, their concomitant occurrence is extremely rare [1, 2]. Spinal SDH comprises 6.5% of all spinal hematomas [3], and the incidence of posterior fossa SDH is 0.01% [4]. Moreover, recorded cases with concomitant spontaneous occurrence of both posterior fossa and spinal SDH with aplastic anemia are extremely rare [5,6,7,8,9]. SDH commonly occurs due to various factors, including vascular malformations, senile brain atrophy, hematological disorders, or iatrogenic factors such drugs side effects [8, 10, 11]. Aplastic anemia is a rare disorder, where patients present with bone marrow suppression, resulting in pancytopenia [12]. The definitive etiology is still unclear, but it is thought to be an immune-based disease that is triggered by some genetic predispositions. Bleeding tendency is one of the hallmarks of this disease because of thrombocytopenia [13]. The present article describes an 11-year-old male who has aplastic anemia with an extremely rare condition, that is, concomitant posterior fossa SDH and spinal SDH.
Case presentation
An 11-year-old boy known to have aplastic anemia presented to our service complaining of headache and persistent vomiting and neck and back pain for 3 days. He had no history of head or spine trauma at all. His parents are relatives “positive consanguinity,” and his sister also suffers from aplastic anemia. Clinical examination at the time of presentation revealed severe pallor with petechial rashes all over the body, with no definite weakness or neurologic or locomotor deficit. Signs of meningeal irritation including neck rigidity and Kernig’s sign were positive. His blood picture indicated pancytopenia (HB = 8 g/dl, WBC = 1.59 10^3/uL, and platelets count = 19.5 10^3/uL). PT, PTT, and other liver function tests were normal. D-dimer and hepatitis B and C serological tests were negative. Bone marrow biopsy presented markedly hypocellular bone marrow with 20% cellularity. Abdominal ultrasonic scan excluded chronic liver disease and hypersplenism.
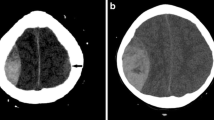
A CT scan of the brain was ordered on emergency bases and showed dilation of the supratentorial ventricles with acute SDH extending over both cerebellar hemispheres and retroclival space. Brain MRI showed posterior fossa and peri-mesencephalic SDH extending anteriorly opposite the whole clivus and posteriorly opposite the surface of both cerebellar hemispheres and associated supratentorial obstructive hydrocephalus. Whole spine MRI showed SDH extending from the lower border of C2 vertebra along the whole spine and down to the level of the lower end of the sacrum associated with spinal cord compression (Fig. 1). A multidisciplinary approach including neurosurgery, pediatric hematology, and clinical pathology team was involved for the management and decision-making of this extremely rare and challenging case.
Initial MRI of the whole craniospinal axis at the time of presentation. A Posterior fossa T1W axial image showing posterior fossa and peri-mesencephalic SDH, B supratentorial T1W axial image showing supratentorial obstructive hydrocephalus, C T1W sagittal image showing SDH extending anteriorly opposite the whole clivus and posteriorly opposite the surface of both cerebellar hemispheres and extending anteriorly to the whole cervical spinal cord, D thoracic spine T1W sagittal image showing SDH extending anteriorly to the thoracic spinal cord, and E lumbosacral T1W sagittal image showing SDH extending posteriorly to the cauda equina down to the level of the lower end of the sacrum and associated with cauda equina compression
After discussion of the pros and cons of different therapeutic options, it has been decided to put the patient under strict observation with conservatives and supporting measures in the ICU. Patient received fluids, steroids, diuretic, and paracetamol infusion. The medical management of aplastic anemia included thrombopoietin receptor agonist TPO-RA; cyclosporin; folic acid; nutritional supplement for vitamins B1, B6, and B12; and Deferasirox. Serial clinical follow-ups were performed for the patient, and his symptoms and signs as well as his hematopoietic parameters showed gradual improvement. He has stayed in the ICU for 10 days, then was discharged to the inpatient ward for a week, then was discharged home from the hospital, and was scheduled for routine outpatient clinic visits. One month after the episode, the patient was clinically symptoms free. A brain and a whole spine MRI were ordered for the patient after 6 months, and they showed complete resolution of the SDH from the whole craniospinal axis with decreased ventricular size (Fig. 2).
Discussion
Concomitant spinal and cranial SDH is a very rare and challenging clinical situation with quiet few cases having been reported in literature. To the best of our knowledge, only 50 cases have been published in literature including only 2 cases of anaplastic anemia [14]. The underlying predisposing factors in such condition could be trauma, vascular malformation, bleeding disorder due to antiplatelet or anticoagulant therapy or thrombocytopenia, bleeding tumor, lumbar puncture, alcohol abuse, or spontaneous [7, 14]. Reporting concomitant spontaneous spinal and cranial SDH in patients with aplastic anemia is very rare with only 2 cases having been reported in literature. In this report, we add to literature the third case (Table 1). The first case was reported in 2008, a 12-year-old male with posterior fossa and spine SDH. The patient was managed conservatively by platelet-rich plasma, mannitol, and steroids [6]. The second case was reported in 2017, a 14-year-old male with bi-frontal, posterior fossa, and spine SDH. The patient was also managed conservatively by multiple blood transfusions and received anti-thymocyte globulins [7].
This case is a known child with history of aplastic anemia who presented with symptoms and signs related to the intracranial component associated with back pain and manifestations of severe aplastic anemia. After careful decision-making by multidisciplinary medical approach, he responded very well to conservative measures and became symptom free within one month and SDH completely resolved on a 6-month MRI.
The specific mechanism for developing the spinal component of the SDH is still unclear as there are no bridging veins in this site in contrast to cranial SDH [10]. There are proposed theories for this condition, and the most accepted one is the blood redistribution from cranial SDH to the spinal SDH, but still there is no proof for this theory. Chronic SDH has membranous boundaries unlike the acute SDH, when acute hemorrhage occurs on top of chronic SDH resulting in the membrane rupture and leakage of the content. Then, by the effect of gravity, SDH can dissect its way along spinal subdural space [8, 11, 15]. Another theory was proposed in patients with ventriculoperitoneal shunts, where low intracranial pressure can cause widening of the subdural space resulting in tears in the bridging veins and opening a tract for SDH to move to the spinal compartment [16]. Furthermore, Ichinose et al. [17] suggested that concomitant cranial and spinal SDH may occur separately as a result of double traumas to the head and the spine.
There are similar published cases with spontaneous concomitant spinal and cranial SDH. Broc-Haro et al. [18] reported a 44-year-old patient with progressive headaches and lumbar radiculopathy. His brain and spine imaging showed frontotemporal SDH and spinal SDH. The patient received supportive therapy for headaches and radicular pain for 2 weeks and showed signs of improvement, and no surgical intervention was required. In addition, Moon et al. [16] reported a 39-year-old patient who was admitted for spinal SDH, and on admission, she started to complain from headache and nausea. Brain CT showed SDH with midline shift that both patients had neither predisposing factor for the SDH nor any history of trauma. The cranial SDH was surgically evacuated due to progressive headaches and to guard against cerebellar herniation. The patient was booked for a later evacuation of the spinal SDH. However, her back and radicular pains improved spontaneously, and no further surgical intervention was required. Lecouvet et al. [11] reported a patient with progressive pain and lower limb weakness. His spine MRI showed spinal SDH. Then, a brain MRI was performed due to the patient’s complaint of severe headaches, and it showed a cortical metastasis with SDH. The brain mass and SDH were surgically evacuated, while the spinal SDH symptoms were well tolerated by the patient and showed spontaneous improvement. Jain et al. [6] conservatively managed their aplastic anemia patient with concomitant cranial and spinal SDH by mannitol, steroids, and platelet rich plasma as a result of the poor general condition and the absence of any neurological deficits, and he had an uneventful recovery course.
Reviewing the reported two children plus our case indicated that this condition occurs in children, who responded very well to conservative therapy and demonstrated good outcome. Consequently, conservative management to address symptoms and treat underlying pathology is recommended in patients with poor general condition and no or mild neurological deterioration. Needless to say, the definitive treatment of aplastic anemia is bone marrow transplantation.
Cranial and spinal SDH is considered neurosurgical emergency especially when rapid neurological deterioration occurs. However, in neurologically stable patients, spontaneous resolution occurs and has been documented [11, 19, 20]. Surgical evacuation is spared for rapidly deteriorating neurological conditions and mainly contributed to the cranial SDH in fear for cerebellar herniation [11, 16]. Spinal SDH can be surgically evacuated in cases of rapidly neurologic deterioration either by open surgery or by minimal invasive lumbar puncture [17]. Most cases of concomitant cranial and spinal SDH had a good recovery spontaneously [8, 11, 21, 22].
Recommendations
Concomitant cranial and spinal SDH requires a multidisciplinary approach, and in patients with predisposing factors, neural axis imaging is required when patients complain of both cranial and spine symptoms. Conservative management involving correcting the cause of bleeding disorder and symptoms relieving agents still has the upper hand in the management except in cases of rapid neurological deterioration in which surgical evacuation is required.
Conclusion
Patients with aplastic anemia or any bleeding disorder conditions should be investigated thoroughly if symptoms denoted a CNS pathology. Concomitant cranial and spinal SDH rarely occurs, and more studies are advocated to be structured to study the specific pathophysiology and etiologies of this condition. Conservative management still has the upper hand. However, surgical intervention for evacuation may be required but in limited situations like progressive and severe neurological deterioration.
Availability of data and materials
All materials used in this article are owned by the authors, and/or no permissions are required.
References
Berhouma M, Houissa S, Jemel H, Khaldi M (2007) Spontaneous chronic subdural hematoma of the posterior fossa. J Neuroradiol = Journal de Neuroradiologie 34:213–215
Kurisu K, Kawabori M, Niiya Y, Ohta Y, Mabuchi S, Houkin K (2012) Bilateral chronic subdural hematomas of the posterior fossae—case report—. Neurol Med Chir (Tokyo) 52:822–825
Kreppel D, Antoniadis G, Seeling W (2003) Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 26:1–49
de Amorim RLO, Stiver SI, Paiva WS, Bor-Seng-Shu E, Sterman-Neto H, de Andrade AF, Teixeira MJ (2014) Treatment of traumatic acute posterior fossa subdural hematoma: report of four cases with systematic review and management algorithm. Acta Neurochir (Wien) 156:199–206
Ahn ES, Smith ER (2005) Acute clival and spinal subdural hematoma with spontaneous resolution: clinical and radiographic correlation in support of a proposed pathophysiological mechanism: case report. J Neurosurg Pediatr 103:175–179
Jain V, Singh J, Sharma R (2008) Spontaneous concomitant cranial and spinal subdural haematomas with spontaneous resolution. Singapore Med J 49:e53–e58
Satyarthee GD, Ahmad F (2018) Spontaneous concurrent intraspinal and intracranial subdural hematoma: management and review of literature. J Pediatr Neurosci 13:24
Yamaguchi S, Kurisu K, Arita K, Takeda M, Tani I, Araki O (2005) Simultaneous cranial and spinal subdural hematoma—case report—. Neurol Med Chir (Tokyo) 45:645–649
Yamasaki M, Akagi K, Niinomi K, Kinoshita S, Kitawaki T, Yoshioka K (1989) Intracranial hemorrhage associated with aplastic anemia. No to hattatsu = Brain Dev 21:215–221
Leber KA, Pendl G, Kogler S, Kammerhuber F, Ebner F (1997) Simultaneous spinal and intracranial chronic subdural hematoma: case illustration. J Neurosurg 87:644
Lecouvet FE, Annet L, Duprez TP, Cosnard G, Scordidis V, Malghem J (2003) Uncommon magnetic resonance imaging observation of lumbar subdural hematoma with cranial origin. J Comput Assist Tomogr 27:530–533
Passweg JR, Marsh JCW (2010) Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematol 2010, Am Soc Hematol Educ Program Book 2010:36–42
Valdez JM, Scheinberg P, Nunez O, Wu CO, Young NS, Walsh TJ (2011) Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis 52:726–735
Akhaddar A (2021) Review of craniospinal acute, subacute, and chronic subdural hematomas. In: Subdural Hematoma. Springer 1–24
Wong ST, Yuen MK, Fok KF, Yuen SC, Yam KY, Fong D (2009) Redistribution of hematoma to spinal subdural space as a mechanism for the rapid spontaneous resolution of posttraumatic intracranial acute subdural hematoma: case report. Surg Neurol 71:99–102
Moon W, Joo W, Chough J, Park H (2013) Spontaneous spinal subdural hematoma concurrent with cranial subdural hematoma. J Korean Neurosurg Soc 54:68
Ichinose D, Tochigi S, Tanaka T, Suzuki T, Takei J, Hatano K, Kajiwara I, Maruyama F, Sakamoto H, Hasegawa Y (2018) Concomitant intracranial and lumbar chronic subdural hematoma treated by fluoroscopic guided lumbar puncture: a case report and literature review. Neurol Med Chir (Tokyo) 58:178–184
Broc-Haro GG, Rodríguez-Valencia F, Manrique-Guzmán S (2008) Acute spontaneous lumbar subdural hematoma associated with subacute cranial subdural hematoma. Case Report Cirugia y Cirujanos 76:161–164
Kang HS, Chung CK, Kim HJ (2000) Spontaneous spinal subdural hematoma with spontaneous resolution. Spinal Cord 38:192–196
Mavroudakis N, Levivier M, Rodesch G (1990) Central cord syndrome due to a spontaneously regressive spinal subdural hematoma. Neurology 40:1306
Hung K-S, Lui C-C, Wang C-H, Wang C-J, Howng S-L (2002) Traumatic spinal subdural hematoma with spontaneous resolution. Spine (Phila Pa 1976) 27:E534–E538
Shimizu S, Tachibana S, Maezawa H, Fujii K, Kan S (1999) Lumbar Spinal Subdural Hematoma Following Craniotomy—Case Report—. Neurol Med Chir (Tokyo) 39:299–301
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ali M Abou-Madawi: conception and design, material preparation, review, and approval of the final manuscript. Mohamed Khaled Elkazaz: first draft of the manuscript and material preparation, data collection, and analysis. Alaa El-Din Saad Abdelhamid: conception and design and final manuscript preparation.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was waived by the local Ethics Committee of Suez Canal University in view of the retrospective nature of the study, and all the procedures being performed were part of the routine care. The study was conducted in accordance with the declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abou-Madawi, A.M., Elkazaz, M.K. & Abdelhamid, A.ED.S. Concomitant spontaneous spinal and posterior fossa subdural hematoma in an 11-year-old child with aplastic anemia: a case report and review of literature. Childs Nerv Syst 38, 2251–2255 (2022). https://doi.org/10.1007/s00381-022-05584-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05584-7