Abstract
Introduction
Craniopharyngiomas are one of the most frequently diagnosed hypothalamo-pituitary tumors in childhood. The adamantinomatous histological subtype accounts for most pediatric cases, while the papillary variant is almost exclusively diagnosed in adults. Here, we report a case of papillary craniopharyngioma in a very young child, confirmed by molecular tissue analysis.
Case report
A 4-year-old girl was being investigated for symptomatic central hypothyroidism. Brain MR imaging revealed a large solid/cystic suprasellar mass, splaying the optic chiasm and measuring 3 × 1.9 × 2.3 cm. The patient underwent a transsphenoidal near total resection of the lesion, which was encased within a tumor capsule. Post-operatively, the patient developed transient diabetes insipidus but otherwise recovered well. The pathology of the lesion was consistent with a papillary craniopharyngioma with regions of stratified squamous epithelium accompanied by superficial goblet cells and ciliated cells. Subsequent next-generation sequencing analysis of the lesion confirmed the presence of a BRAF V600E mutation (BRAFc.1799T>A p. (Val600Glu). To date, she remains free from progression 1 year following surgery.
Conclusion
This is the youngest case published to date of papillary craniopharyngioma with a confirmed BRAF V600E mutation. The case encourages discussion about the most appropriate adjuvant therapy for tumor progression in such cases, given the risks of radiotherapy to the developing brain and the increasing availability of oral BRAF inhibitor therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas are frequently diagnosed hypothalamo-pituitary tumors of childhood [12]. Despite a benign histology, their management presents an ongoing challenge given lesional proximity to critical structures. Disease progression or surgical intervention can result in neuro-endocrinological symptoms, visual disturbances, and obstructive hydrocephalus, which impacts on quality of life [9, 13, 22]. The apparent futility of chemotherapy [10], and concerns regarding adverse sequelae from adjuvant radiotherapy, particularly for the developing brain, compound the dilemma [14, 20].
Two histopathological variants are recognized [17]. Adamantinomatous craniopharyngiomas account for most pediatric cases [23]. Lesions often consist of cystic elements and are characterized by distinctive epithelial appearances featuring dense whorls merged with looser stellate reticulum, cholesterol crystals, and wet keratin [17]. In contrast, squamous papillary craniopharyngiomas are almost exclusive to adults. They are typically solid and homogenous in composition, comprising well-differentiated squamous epithelium without calcification, whorls, or wet keratin. Biological distinctions are also evident; WNT pathway aberrations are associated with adamantinomatous craniopharyngiomas [11], while up to 95% of papillary lesions harbor BRAF V600E mutations [6].
Here, we report one of the youngest patients to date with a papillary craniopharyngioma, confirmed by histological and molecular analysis. We demonstrate its rarity by literature review and discuss adjuvant therapeutic implications for affected young children, given the concerns regarding conventional radiotherapy and the advent of novel targeted therapy.
Case report
A 4-year-old girl presented with symptomatic central hypothyroidism and short stature. Initial examination was unremarkable, including normal visual field assessment on confrontation testing.
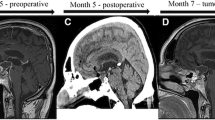
Assessment of pituitary functioning confirmed central hypothyroidism, but also identified cortisol and growth hormone deficiency. Subsequent gadolinium-enhanced magnetic resonance imaging (MRI) of the brain identified a solid/cystic suprasellar lesion, measuring 3 × 1.9 × 2.3 cm (Fig. 1a). The superior, solid aspect demonstrated increased T1 signal, while high FLAIR signal and peripheral contrast enhancement were noted in the more cystic, inferior component. The optic chiasm was splayed over the lesion. The ventricular system was normal.
Sagittal pre-operative post-contrast T1 MRI brain scan image at presentation (a) revealing a large suprasellar lesion, measuring 3 × 1.9 × 2.3 cm. The lesion compromised a superior, solid component which had increased T1 signal pre-contrast (solid arrow head). This component also demonstrated high T2/FLAIR signal. An inferior, low FLAIR signal cystic component was noted. Peripheral rim enhancement of the lesion can be appreciated (white open arrow). b is a coronal T2 image also obtained at presentation which shows the upwards extend of the lesion (solid arrow) impinging onto and splaying the optic chiasm (open white arrow). Following transsphenoidal tumor surgery, follow-up sagittal T1 post-contrast MRI imaging (c) demonstrates improved appearances, with a small degree of non-enhancing residual tissue extending down into an enlarged pituitary sella (white arrow). The coronal T1 post-contrast image (d) now demonstrates a normal position of the optic chiasm (solid arrow) and a clearly discernable pituitary stalk which is slightly deviated to the left (white open arrow)
The patient underwent a transsphenoidal near total resection of the mass, which was identified within a thick capsule, atypical for an adamantinomatous craniopharyngioma. Opening the lesion revealed a necrotic, cream-like material which was removed internally by suction and curettage. The residual cyst wall was unable to be completely dissected from surrounding structures.
The patient recovered well post-operatively, developing only transient diabetes insipidus which resolved within days. She had no neurological deficits following resection and post-operative visual field testing was comparable to presentation. The patient subsequently commenced levothyroxine and hydrocortisone therapy, while growth hormone replacement was planned to commence once clinical and radiological stability were confirmed.
Post-operative MRI scans showed residual enhancing cyst wall but no measurable solid component (Fig. 1b). To date, the patient remains clinically and radiologically stable with no evidence of disease progression, 11 months following surgery.
Histopathological analysis of the lesion demonstrated stratified squamous epithelium accompanied by superficial goblet cells and ciliated cells. Underlying tissue stroma comprised loose connective tissue and blood vessels. No wet keratin was identified. The epithelial cells demonstrated physiological, membranous beta-catenin staining, thereby lacking evidence for Wnt pathway activation. Suprabasal epithelial cells stained positive for CK7 and superficial epithelial cells demonstrated strong CAM5.2 positivity. CK20 staining was negative. The morphological appearances were therefore consistent with a diagnosis of papillary craniopharyngioma (Fig. 2a, b). Biological re-affirmation was sought using next-generation sequencing analysis of the lesion, which confirmed a BRAF V600E mutation (BRAFc.1799T>A p. (Val600Glu)) thereby validating the histopathological diagnosis.
Hematoxylin and Eosin staining at × 10 magnification (a). Histological assessment revealed fragments of stratified squamous epithelium and an absence of wet keratin. An acute inflammatory cell infiltrate was observed throughout the tissue. High power magnification (× 40; b) allows appreciation of both ciliated cells (black arrow) and goblet cells (white asterisk). The underlying stroma was composed of loose connective tissue and blood vessels. Immunohistochemistry revealed an absence of intranuclear Beta-catenin staining (a feature of adamantinomatous craniopharyngiomas)
Discussion
To our knowledge, this is the youngest patient published to date with a papillary craniopharyngioma and confirmed BRAF V600E mutation.
The main differential diagnoses included adamantinomatous craniopharyngioma, Langerhans cell histiocytosis (LCH), and Rathke’s cleft cyst (RCC) with squamous metaplasia. Histological and clinical assessment discounted LCH, while the absence of Wnt pathway activation excluded an adamantinomatous craniopharyngioma. Likewise, despite sharing histological similarities to a papillary craniopharyngioma, RCC was excluded by the absence of a BRAF point mutation [15, 19, 27]. Whether a RCC can acquire a BRAF mutation and transform into a papillary craniopharyngioma remains hypothetical [26].
Papillary craniopharyngiomas are extremely rare in children. This is highlighted by a literature review of published pediatric cases (aged below 16 years), using OVID and Pubmed search with clearly defined terms (Table 1). None of the published cases identified had complete clinical information, such that the effectiveness of conventional management strategies for this patient group could not be ascertained.
Our patient remains stable almost 1 year following tumor surgery. Nevertheless, in adults, papillary craniopharyngiomas are often refractory to initial treatment. Therefore, the case encourages discussion on the most appropriate adjuvant therapy for young children with papillary craniopharyngiomas who have significant post-operative tumor residuum or progression. While stereotactic radiotherapy remains the recognized standard, concerns persist regarding neurocognitive, vascular, metabolic, and endocrinological sequelae, even with state-of-the-art proton beam radiotherapy [14, 20, 21].
Moreover, since most papillary craniopharyngiomas harbor the BRAF V600E oncogenic mutation [6], a novel alternative is drug inhibition of the encompassing mitogen-activated protein kinase (MAPK) pathway controlling cell division, differentiation, and invasion. BRAF inhibitors, including vemurafenib and dabrafenib, have proved successful either alone or in conjunction with trametinib, an inhibitor of another MAPK member MEK, to treat adults with papillary craniopharyngioma, resulting in tumor shrinkage with good tolerability [2, 5, 24, 25]. In addition, increasing pediatric data is emerging for BRAF and MEK inhibitors in the treatment of surgically inaccessible gliomas that express BRAF V600E mutations, with an acceptable side effect profile in this age group [1, 3, 4, 16, 18, 29]. While initial results are promising, long-term safety data is lacking as is knowledge on the longevity of therapy required.
Conclusion
This case represents the youngest patient reported to date with a papillary craniopharyngioma that has been validated by molecular analysis, revealing a BRAF V600E mutation. While a rare occurrence, such a molecular test, is advocated if, following histopathological analysis, papillary craniopharyngioma and Rathke’s cleft cyst remain possible diagnoses. Given the burgeoning data on the efficacy and tolerability of MAPK pathway drug inhibition, this adjuvant therapeutic option warrants consideration against conventional stereotactic radiotherapy for recurrent or refractory residual pediatric papillary craniopharyngiomas.
References
Aguilera D, Janss A, Mazewski C, Castellino RC, Schniederjan M, Hayes L, Brahma B, Fogelgren L, MacDonald TJ (2016) Successful retreatment of a child with a refractory brainstem ganglioglioma with vemurafenib. Pediatr Blood Cancer 63:541–543. https://doi.org/10.1002/pbc.25787
Aylwin SJ, Bodi I, Beaney R (2016) Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary 19:544–546. https://doi.org/10.1007/s11102-015-0663-4
Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, Prados M, Packer RJ, Qaddoumi I, Gururangan S, Pollack IF, Goldman S, Doyle LA, Stewart CF, Boyett JM, Kun LE, Fouladi M (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro-Oncology 19:1135–1144. https://doi.org/10.1093/neuonc/now282
Bavle A, Jones J, Lin FY, Malphrus A, Adesina A, Su J (2017) Dramatic clinical and radiographic response to BRAF inhibition in a patient with progressive disseminated optic pathway glioma refractory to MEK inhibition. Pediatr Hematol Oncol 34:254–259. https://doi.org/10.1080/08880018.2017.1360971
Brastianos PK, Shankar GM, Gill CM, Taylor-Weiner A, Nayyar N, Panka DJ, Sullivan RJ, Frederick DT, Abedalthagafi M, Jones PS, Dunn IF, Nahed BV, Romero JM, Louis DN, Getz G, Cahill DP, Santagata S, Curry WT, Jr., Barker FG, 2nd (2016) Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst 108. https://doi.org/10.1093/jnci/djv310
Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, Dias-Santagata D, Thorner AR, Lawrence MS, Rodriguez FJ, Bernardo LA, Schubert L, Sunkavalli A, Shillingford N, Calicchio ML, Lidov HG, Taha H, Martinez-Lage M, Santi M, Storm PB, Lee JY, Palmer JN, Adappa ND, Scott RM, Dunn IF, Laws ER Jr, Stewart C, Ligon KL, Hoang MP, Van Hummelen P, Hahn WC, Louis DN, Resnick AC, Kieran MW, Getz G, Santagata S (2014) Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 46:161–165. https://doi.org/10.1038/ng.2868
Cheng J, Shao Q, Pan Z, You J (2016) Analysis and long-term follow-up of the surgical treatment of children with craniopharyngioma. J Craniofac Surg 27:e763–e766. https://doi.org/10.1097/SCS.0000000000003176
Crotty TB, Scheithauer BW, Young WF Jr, Davis DH, Shaw EG, Miller GM, Burger PC (1995) Papillary craniopharyngioma: a clinicopathological study of 48 cases. J Neurosurg 83:206–214. https://doi.org/10.3171/jns.1995.83.2.0206
Gautier A, Godbout A, Grosheny C, Tejedor I, Coudert M, Courtillot C, Jublanc C, De Kerdanet M, Poirier JY, Riffaud L, Sainte-Rose C, Van Effenterre R, Brassier G, Bonnet F, Touraine P, Craniopharyngioma Study G (2012) Markers of recurrence and long-term morbidity in craniopharyngioma: a systematic analysis of 171 patients. J Clin Endocrinol Metab 97:1258–1267. https://doi.org/10.1210/jc.2011-2817
Hargrave DR (2006) Does chemotherapy have a role in the management of craniopharyngioma? J Pediatr Endocrinol Metab 19(Suppl 1):407–412
Holsken A, Sill M, Merkle J, Schweizer L, Buchfelder M, Flitsch J, Fahlbusch R, Metzler M, Kool M, Pfister SM, von Deimling A, Capper D, Jones DT, Buslei R (2016) Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun 4:20. https://doi.org/10.1186/s40478-016-0287-6
Jane JA Jr, Laws ER (2006) Craniopharyngioma. Pituitary 9:323–326. https://doi.org/10.1007/s11102-006-0413-8
Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, Marymont MH (2003) Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol 40:214–218. https://doi.org/10.1002/mpo.10247
Kiehna EN, Merchant TE (2010) Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus 28:E10. https://doi.org/10.3171/2010.1.FOCUS09297
Kim JH, Paulus W, Heim S (2015) BRAF V600E mutation is a useful marker for differentiating Rathke’s cleft cyst with squamous metaplasia from papillary craniopharyngioma. J Neuro-Oncol 123:189–191. https://doi.org/10.1007/s11060-015-1757-6
Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, Zapotocky M, McKeown T, Hawkins C, Bouffet E, Tabori U (2016) Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer 63:2038–2041. https://doi.org/10.1002/pbc.26086
Louis D, Ohgaki, H., Wiestler, O., Cavenee, W., Ellison, D., Figarella-Branger, D., (2016) WHO Classification of Tumours of the Central Nervous System, Revised, 4th Edn
Marks AM, Bindra RS, DiLuna ML, Huttner A, Jairam V, Kahle KT, Kieran MW (2018) Response to the BRAF/MEK inhibitors dabrafenib/trametinib in an adolescent with a BRAF V600E mutated anaplastic ganglioglioma intolerant to vemurafenib. Pediatr Blood Cancer 65:e26969. https://doi.org/10.1002/pbc.26969
Marucci G, de Biase D, Zoli M, Faustini-Fustini M, Bacci A, Pasquini E, Visani M, Mazzatenta D, Frank G, Tallini G (2015) Targeted BRAF and CTNNB1 next-generation sequencing allows proper classification of nonadenomatous lesions of the sellar region in samples with limiting amounts of lesional cells. Pituitary 18:905–911. https://doi.org/10.1007/s11102-015-0669-y
Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, Boop FA, Sanford RA (2006) Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg 104:94–102. https://doi.org/10.3171/ped.2006.104.2.5
Mizumoto M, Oshiro Y, Yamamoto T, Kohzuki H, Sakurai H (2017) Proton beam therapy for pediatric brain tumor. Neurol Med Chir (Tokyo) 57:343–355. https://doi.org/10.2176/nmc.ra.2017-0003
Muller HL (2013) Childhood craniopharyngioma. Pituitary 16:56–67. https://doi.org/10.1007/s11102-012-0401-0
Pekmezci M, Louie J, Gupta N, Bloomer MM, Tihan T (2010) Clinicopathological characteristics of adamantinomatous and papillary craniopharyngiomas: University of California, San Francisco experience 1985-2005. Neurosurgery 67:1341–1349; discussion 1349. https://doi.org/10.1227/NEU.0b013e3181f2b583
Roque A, Odia Y (2017) BRAF-V600E mutant papillary craniopharyngioma dramatically responds to combination BRAF and MEK inhibitors. CNS Oncol 6:95–99. https://doi.org/10.2217/cns-2016-0034
Rostami E, Witt Nystrom P, Libard S, Wikstrom J, Casar-Borota O, Gudjonsson O (2017) Recurrent papillary craniopharyngioma with BRAFV600E mutation treated with neoadjuvant-targeted therapy. Acta Neurochir 159:2217–2221. https://doi.org/10.1007/s00701-017-3311-0
Schlaffer SM, Buchfelder M, Stoehr R, Buslei R, Holsken A (2018) Rathke’s cleft cyst as origin of a pediatric papillary craniopharyngioma. Front Genet 9:49. https://doi.org/10.3389/fgene.2018.00049
Schweizer L, Capper D, Holsken A, Fahlbusch R, Flitsch J, Buchfelder M, Herold-Mende C, von Deimling A, Buslei R (2015) BRAF V600E analysis for the differentiation of papillary craniopharyngiomas and Rathke’s cleft cysts. Neuropathol Appl Neurobiol 41:733–742. https://doi.org/10.1111/nan.12201
Tariq MU, Din NU, Ahmad Z, Memon W (2017) Papillary craniopharyngioma: a clinicopathologic study of a rare entity from a major tertiary care center in Pakistan. Neurol India 65:570–576. https://doi.org/10.4103/neuroindia.NI_552_16
Wagner LM, Myseros JS, Lukins DE, Willen CM, Packer RJ (2018) Targeted therapy for infants with diencephalic syndrome: a case report and review of management strategies. Pediatr Blood Cancer 65:e26917. https://doi.org/10.1002/pbc.26917
Zhang YQ, Wang CC, Ma ZY (2002) Pediatric craniopharyngiomas: clinicomorphological study of 189 cases. Pediatr Neurosurg 36:80–84. https://doi.org/10.1159/000048357
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed parental consent was obtained from the individual included in the study.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Borrill, R., Cheesman, E., Stivaros, S. et al. Papillary craniopharyngioma in a 4-year-old girl with BRAF V600E mutation: a case report and review of the literature. Childs Nerv Syst 35, 169–173 (2019). https://doi.org/10.1007/s00381-018-3925-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3925-4