Abstract
Purpose
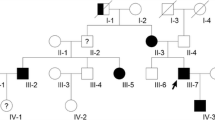
Cerebrospinal cavernous malformations (CCMs) are vascular lesions characterized by dilated and leaky capillary caverns. CCMs can cause seizures, focal neurological deficits or acute intracranial hemorrhage; however, most patients are asymptomatic. CCMs occur either sporadically or as a familial autosomal-dominant disorder. We present a clinical and molecular study of a patient with distinctive cerebral and spinal cavernous malformations following radiochemotherapy for a malignant brain tumor.
Methods
The patient had multiple magnet resonance imaging (MRI) examinations of his brain and spine following radiochemotherapy for a primary intracranial germ cell tumor (GCT), as part of his oncologic follow-up. The MRI sequences included susceptibility-weighted imaging (SWI). The coding exons and their flanking intronic regions of KRIT1/CCM1 gene were analyzed for mutations by polymerase chain reaction (PCR) and direct sequencing.
Results
MRI revealed numerous cerebral and spinal microhemorrhages and pronounced cavernous malformations that progressed with subsequent follow-up imaging. Genetic analysis demonstrated a novel heterozygous KRIT1/CCM1 two base pair deletion (c.1535_1536delTG) in exon 14. This deletion leads to a frameshift with a premature stop codon at nucleotide position 1553 and a highly likely loss of function of the KRIT1 protein.
Conclusion
We describe a patient with a novel heterozygous germ line loss of function mutation in KRIT1, which is associated with rapid-onset and highly progressive CCMs after radiochemotherapy for a malignant brain tumor.





Similar content being viewed by others
References
Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA (2009) Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet 18:919–930. doi:10.1093/hmg/ddn430
Allen JC, Miller DC, Budzilovich GN, Epstein FJ (1991) Brain and spinal cord hemorrhage in long-term survivors of malignant pediatric brain tumors: a possible late effect of therapy. Neurology 41:148–150
Arrieta O, Michel Ortega RM, Angeles-Sanchez J, Villarreal-Garza C, Aviles-Salas A, Chanona-Vilchis JG, Arechaga-Ocampo E, Luevano-Gonzalez A, Jimenez MA, Aguilar JL (2009) Serum human chorionic gonadotropin is associated with angiogenesis in germ cell testicular tumors. Journal of experimental & clinical cancer research : CR 28:120. doi:10.1186/1756-9966-28-120
Azzam EI, Jay-Gerin JP, Pain D (2012) Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 327:48–60. doi:10.1016/j.canlet.2011.12.012
Batra S, Lin D, Recinos PF, Zhang J, Rigamonti D (2009) Cavernous malformations: natural history, diagnosis and treatment. Nat Rev Neurol 5:659–670. doi:10.1038/nrneurol.2009.177
Baumgartner JE, Ater JL, Ha CS, Kuttesch JF, Leeds NE, Fuller GN, Wilson RJ (2003) Pathologically proven cavernous angiomas of the brain following radiation therapy for pediatric brain tumors. Pediatr Neurosurg 39:201–207 doi:72472
Bulut HT, Sarica MA, Baykan AH (2014) The value of susceptibility weighted magnetic resonance imaging in evaluation of patients with familial cerebral cavernous angioma. Int J Clin Exp Med 7:5296–5302
Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garre ML, Patte C, Ricardi U, Saran F, Frappaz D (2013) SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro-Oncology 15:788–796. doi:10.1093/neuonc/not019
Cave-Riant F, Denier C, Labauge P, Cecillon M, Maciazek J, Joutel A, Laberge-Le Couteulx S, Tournier-Lasserve E (2002) Spectrum and expression analysis of KRIT1 mutations in 121 consecutive and unrelated patients with cerebral cavernous malformations. European journal of human genetics : EJHG 10:733–740. doi:10.1038/sj.ejhg.5200870
Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ Jr, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Tsukita S, Hoover KB et al (1998) The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 23:281–282
Choquet H, Pawlikowska L, Lawton MT, Kim H (2015) Genetics of cerebral cavernous malformations: current status and future prospects. J Neurosurg Sci 59:211–220
Clatterbuck RE, Elmaci I, Rigamonti D (2001) The nature and fate of punctate (type IV) cavernous malformations. Neurosurgery 49:26–30 discussion 30-22
Cutsforth-Gregory JK, Lanzino G, Link MJ, Brown RD Jr, Flemming KD (2015) Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg 122:1214–1222. doi:10.3171/2015.1.JNS141452
Denier C, Labauge P, Bergametti F, Marchelli F, Riant F, Arnoult M, Maciazek J, Vicaut E, Brunereau L, Tournier-Lasserve E (2006) Genotype-phenotype correlations in cerebral cavernous malformations patients. Ann Neurol 60:550–556. doi:10.1002/ana.20947
DiStefano PV, Kuebel JM, Sarelius IH, Glading AJ (2014) KRIT1 protein depletion modifies endothelial cell behavior via increased vascular endothelial growth factor (VEGF) signaling. J Biol Chem 289:33054–33065. doi:10.1074/jbc.M114.582304
Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E (2013) Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med 19:302–308. doi:10.1016/j.molmed.2013.02.004
Francalanci F, Avolio M, De Luca E, Longo D, Menchise V, Guazzi P, Sgro F, Marino M, Goitre L, Balzac F, Trabalzini L, Retta SF (2009) Structural and functional differences between KRIT1A and KRIT1B isoforms: a framework for understanding CCM pathogenesis. Exp Cell Res 315:285–303. doi:10.1016/j.yexcr.2008.10.006
Gastelum E, Sear K, Hills N, Roddy E, Randazzo D, Chettout N, Hess C, Cotter J, Haas-Kogan DA, Fullerton H, Mueller S (2015) Rates and characteristics of radiographically detected intracerebral cavernous malformations after cranial radiation therapy in pediatric cancer patients. J Child Neurol 30:842–849. doi:10.1177/0883073814544364
Gault J, Shenkar R, Recksiek P, Awad IA (2005) Biallelic somatic and germ line CCM1 truncating mutations in a cerebral cavernous malformation lesion. Stroke 36:872–874. doi:10.1161/01.STR.0000157586.20479.fd
Glading A, Han J, Stockton RA, Ginsberg MH (2007) KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol 179:247–254. doi:10.1083/jcb.200705175
Goitre L, De Luca E, Braggion S, Trapani E, Guglielmotto M, Biasi F, Forni M, Moglia A, Trabalzini L, Retta SF (2014) KRIT1 loss of function causes a ROS-dependent upregulation of c-Jun. Free Radic Biol Med 68:134–147. doi:10.1016/j.freeradbiomed.2013.11.020
Golden M, Saeidi S, Liem B, Marchand E, Morrison L, Hart B (2015) Sensitivity of patients with familial cerebral cavernous malformations to therapeutic radiation. Journal of medical imaging and radiation oncology 59:134–136. doi:10.1111/1754-9485.12269
Greenfield JG, Love S, Budka H, Perry A (2015) Greenfield's neuropathology, Ninth edn. CRC Press, London
Guzeloglu-Kayisli O, Kayisli UA, Amankulor NM, Voorhees JR, Gokce O, DiLuna ML, Laurans MS, Luleci G, Gunel M (2004) Krev1 interaction trapped-1/cerebral cavernous malformation-1 protein expression during early angiogenesis. J Neurosurg 100:481–487. doi:10.3171/ped.2004.100.5.0481
Heckl S, Aschoff A, Kunze S (2002) Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer 94:3285–3291. doi:10.1002/cncr.10596
Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S (2005) Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol 26:1158–1162
Keezer MR, Del Maestro R (2009) Radiation-induced cavernous hemangiomas: case report and literature review. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques 36:303–310
Kleinschmidt-DeMasters B, Rodríguez FJ, Tihan T (2016) Diagnostic pathology. neuropathology, Second edn. Elsevier, Philadelphia
Knudson AG Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 68:820–823
Koike S, Aida N, Hata M, Fujita K, Ozawa Y, Inoue T (2004) Asymptomatic radiation-induced telangiectasia in children after cranial irradiation: frequency, latency, and dose relation. Radiology 230:93–99. doi:10.1148/radiol.2301021143
Labauge P, Denier C, Bergametti F, Tournier-Lasserve E (2007) Genetics of cavernous angiomas. Lancet Neurol 6:237–244. doi:10.1016/S1474-4422(07)70053-4
Laberge-le Couteulx S, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M, Marechal E, Joutel A, Bach JF, Tournier-Lasserve E (1999) Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet 23:189–193. doi:10.1038/13815
Larson JJ, Ball WS, Bove KE, Crone KR, Tew JM Jr (1998) Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. J Neurosurg 88:51–56. doi:10.3171/jns.1998.88.1.0051
Li L, Mugikura S, Kumabe T, Murata T, Mori E, Takase K, Jingu K, Takahashi S (2015) A comparative study of the extent of cerebral microvascular injury following whole-brain irradiation versus reduced-field irradiation in long-term survivors of intracranial germ cell tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 117:302–307. doi:10.1016/j.radonc.2015.09.017
Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. Journal of magnetic resonance imaging : JMRI 42:23–41. doi:10.1002/jmri.24768
McDonald DA, Shi C, Shenkar R, Gallione CJ, Akers AL, Li S, De Castro N, Berg MJ, Corcoran DL, Awad IA, Marchuk DA (2014) Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Hum Mol Genet 23:4357–4370. doi:10.1093/hmg/ddu153
Morris B, Partap S, Yeom K, Gibbs IC, Fisher PG, King AA (2009) Cerebrovascular disease in childhood cancer survivors: a children's oncology group report. Neurology 73:1906–1913. doi:10.1212/WNL.0b013e3181c17ea8
Pagenstecher A, Stahl S, Sure U, Felbor U (2009) A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet 18:911–918. doi:10.1093/hmg/ddn420
Passos J, Nzwalo H, Marques J, Azevedo A, Netto E, Nunes S, Salgado D (2015) Late cerebrovascular complications after radiotherapy for childhood primary central nervous system tumors. Pediatr Neurol 53:211–215. doi:10.1016/j.pediatrneurol.2015.05.015
Perry A, Brat DJ (2010) Practical surgical neuropathology : a diagnostic approach. Pattern recognition series. Churchill Livingstone/Elsevier, Philadelphia.
Peters S, Pahl R, Claviez A, Jansen O (2013) Detection of irreversible changes in susceptibility-weighted images after whole-brain irradiation of children. Neuroradiology 55:853–859. doi:10.1007/s00234-013-1185-2
Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA (2004) Loss of p53 sensitizes mice with a mutation in Ccm1 (KRIT1) to development of cerebral vascular malformations. Am J Pathol 165:1509–1518. doi:10.1016/S0002-9440(10)63409-8
Raychaudhuri R, Batjer HH, Awad IA (2005) Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol 63:319–328; discussion 328. doi:10.1016/j.surneu.2004.05.032
Reisinger K, Baal N, McKinnon T, Munstedt K, Zygmunt M (2007) The gonadotropins: tissue-specific angiogenic factors? Mol Cell Endocrinol 269:65–80. doi:10.1016/j.mce.2006.11.015
Retta SF, Glading AJ (2016) Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: two sides of the same coin. Int J Biochem Cell Biol. doi:10.1016/j.biocel.2016.09.011
Riant F, Bergametti F, Ayrignac X, Boulday G, Tournier-Lasserve E (2010) Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J 277:1070–1075. doi:10.1111/j.1742-4658.2009.07535.x
Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF (1987) The MRI appearance of cavernous malformations (angiomas). J Neurosurg 67:518–524. doi:10.3171/jns.1987.67.4.0518
Robbins ME, Zhao W (2004) Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol 80:251–259. doi:10.1080/09553000410001692726
Robinson JR, Awad IA, Little JR (1991) Natural history of the cavernous angioma. J Neurosurg 75:709–714. doi:10.3171/jns.1991.75.5.0709
Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA (1997) Association of Krev-1/rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21-22. Oncogene 15:1043–1049. doi:10.1038/sj.onc.1201268
Strenger V, Sovinz P, Lackner H, Dornbusch HJ, Lingitz H, Eder HG, Moser A, Urban C (2008) Intracerebral cavernous hemangioma after cranial irradiation in childhood. Incidence and risk factors Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft 184:276–280. doi:10.1007/s00066-008-1817-3
Verlaan DJ, Davenport WJ, Stefan H, Sure U, Siegel AM, Rouleau GA (2002) Cerebral cavernous malformations: mutations in Krit1. Neurology 58:853–857
Wustehube J, Bartol A, Liebler SS, Brutsch R, Zhu Y, Felbor U, Sure U, Augustin HG, Fischer A (2010) Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc Natl Acad Sci U S A 107:12640–12645. doi:10.1073/pnas.1000132107
Zabramski JM, Henn JS, Coons S (1999) Pathology of cerebral vascular malformations. Neurosurg Clin N Am 10:395–410
Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, Brown B, Rigamonti D, Brown G (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80:422–432. doi:10.3171/jns.1994.80.3.0422
Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA (2002) KRIT1 association with the integrin-binding protein ICAP-1: a new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Hum Mol Genet 11:389–396
Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT (2002) Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab 87:5290–5296. doi:10.1210/jc.2002-020642
Acknowledgements
We sincerely thank the patient and his family for participating in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. 6
CCM presentation in different MRI sequences. Seven years and 4 months after completion of initial radiotherapy, MRI scans through a similar axial level of the brain show various appearances of the CCMs. Comparison of T1-weighted (a), T2-weighted (b, c) and T2*-weighted (d) images highlight the sensitivity of the different signal sequences in detection of CCMs. (GIF 109 kb)
Rights and permissions
About this article
Cite this article
Russo, A., Neu, M.A., Theruvath, J. et al. Novel loss of function mutation in KRIT1/CCM1 is associated with distinctly progressive cerebral and spinal cavernous malformations after radiochemotherapy for intracranial malignant germ cell tumor. Childs Nerv Syst 33, 1275–1283 (2017). https://doi.org/10.1007/s00381-017-3434-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3434-x