Abstract
Purpose
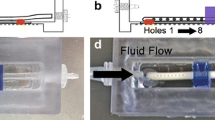
Ventricular catheter drainage holes of shunt systems used to treat hydrocephalus obstruct with tissue commonly comprising monocytes/macrophages, astrocytes, and giant cells. Despite high rates of obstruction, very few studies have manipulated drainage hole orientation, number, position, or diameter. By altering the hole diameter but maintaining a constant hole surface area, we manipulated shear stress through the holes, which we hypothesized would change the degree of macrophage and astrocyte attachment.
Methods
First, a hole fabrication method was chosen from two fabrication techniques including punched holes in catheter tubing and constructed holes using nanofabrication techniques.
Results
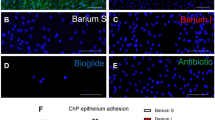
Punched holes were chosen to vary hole size from 282 to 975 μm because (1) samples were geometrically similar to commercially available ventricular catheters without significant microscopic differences in roughness values and (2) total macrophage and astrocyte adhesion on the punched holes was not significantly different from adhesion on the commercially available catheters. Overall adhesion from least to most adherent appeared to follow 975 < 754 ≈ 500 < 282-μm hole diameter for macrophages and 975 < 500 < 754 < 282 for astrocytes with an obvious dependency on catheter orientation with respect to the horizontal; a dependency to the proximity of the hole to the catheter tip was not observed.
Conclusion
This study suggests that macrophage and astrocyte adhesion generally decreases with increasing hole diameter under flow conditions and underscores the necessity for future work to examine how hole diameter impacts inflammatory-based shunt obstruction.









Similar content being viewed by others
References
Abramoff M (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Bigner S, Elmore P, Dee A, Johnston W (1985) The cytopathology of reactions to ventricular shunts. Acta Cytol 29:391–396
Campbell C, Von Recum A (1989) Microtopography and soft tissue response. J Investig Surg 2:51–74
Del Bigio M, Bruni J (1986) Reaction of rabbit lateral periventricular tissue to shunt tubing implants. J Neurosurg 64:932–940
Duffy D, McDonald J, Schueller O, Whitesides G (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984
Giang U, Lee D, King M, DeLouise L (2007) Microfabrication of cavities in polydimethylsiloxane using DRIE silicon molds. Lab Chip 7:1660–1662
Ginsberg H, Sum A, Drake J, Cobbold R (2000) Ventriculoperitoneal shunt flow dependency on the number of patent holes in a ventricular catheter. Pediatr Neurosurg 33:7–11
Ginsberg H, Drake J, Peterson T, Cobbold R (2006) Recanalization of obstructed cerebrospinal fluid ventricular catheters using ultrasonic cavitation. Neurosurgery 59:ONS403-412
Hallab N, Bundy K, O’Connor K, Clark R, Moses R (1995) Cell adhesion to biomaterials: correlations between surface charge, surface roughness, adsorbed protein, and cell morphology. J Long Term Eff Med Implants 5:209–231
Harris C, Resau J, Hudson E, West R, Moon C, McAllister J (2010) Mechanical contributions to astrocyte adhesion using a novel in vitro model of catheter obstruction. Exp Neurol 222:204–210
Harris C, Resau J, Hudson E, West R, Moon C, Black A, McAllister JP (2011) Effects of surface wettability, flow and protein concentration on macrophage and astrocyte adhesion in an in vitro model of central nervous system catheter obstruction. J Biomed Mater Res A (in press)
Jenney C, DeFife K, Colton E, Anderson J (1998) Human monocyte/macrophage adhesion, macrophage motility, and IL-4-induced foreign body giant cell formation on silane-modified surface in vitro. J Biomed Mater Res 41:171–184
Klinge U, Klosterhalfen B, Birkenhauer V, Junge K, Conze J, Schumpelick V (2002) Impact of polymer pore size on the interface scar formation in a rat model. J Surg Res 103:208–214
Lin J, Morris M, Olivero W, Boop F, Sanford R (2003) Computational and experimental study of proximal flow in ventricular catheters. Technical note. J Neurosurg 99:426–431
Lintula L (2008) How shunts are made. It’s about life: 10th National Conference on Hydrocephalus, Park City, Utah
Long M, SLu S, Sun G (2006) Kinetics of receptor–ligand interactions in immune responses. Cell Mol Immunol 3:79–86
Mata A, Fleischman A, Roy S (2005) Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices 7:281–293
Medtronic Inc. (2006) Rivulet. United States Patent Serial No. 78887628
Schoener W (1991) Evaluation of shunt failures by compliance analysis and inspection of shunt valves and shunt materials, using microscopic or scanning electron microscopic techniques. In: Matsumoto S, Tamaki N (eds) Hydrocephalus: pathogenesis and treatment. Springer, Tokyo, pp 452–472
Sekhar L, Moossy J, Guthkelch A (1982) Malfunctioning ventriculoperitoneal shunts. Clinical and pathological features. J Neurosurg 56:411–416
Silverberg G, Huhn S, Jaffe R, Chang S, Saul T, Heit G, Von Essen A, Rubenstein E (2002) Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. J Neurosurg 97:1271–1275
Thomale (2010) Performation holes in ventricular catheters—is less more? Childs Nerv Syst 26:781–789
Zhu C, Yago T, Lou J, Zarnitsyna V, McEver R (2008) Mechanisms for flow-enhanced cell adhesion. Ann Biomed Eng 36:604–621
Acknowledgments
We are grateful for the encouragement and suggestions provided by Marion Walker, MD, and John Kestle, MD, as well as technical aid provided by Daniel Harris, MS, and Brian Baker. We thank Kristin Kraus, MS for editorial assistance. Funding was provided by the Division of Pediatric Neurosurgery, Primary Children’s Medical Center, the Department of Neurosurgery at the University of Utah School of Medicine, and STARS-kids (Seeking Techniques Advancing Research in Shunts).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harris, C.A., McAllister, J.P. Does drainage hole size influence adhesion on ventricular catheters?. Childs Nerv Syst 27, 1221–1232 (2011). https://doi.org/10.1007/s00381-011-1430-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-011-1430-0