Abstract
Purpose
Shunt valves, required for treatment of hydrocephalus, demand for high performance rates and lifelong excellent function. To overcome problems with traditional silicone materials, adjustable and gravity-adapted titanium valves were developed. Even modern shunt valve systems are still subject to occlusion. The aim of the present study was to investigate dysfunctional silicone and titanium valves for presence of cellular and proteinous materials inside the housings by means of histopathology.
Methods
A total of 19 explanted shunt valves from children between 2 and 182 months of age were investigated following dysfunction. After fixation in formalin and embedding in hard resin, slices were ground to a thickness of 5–30 μ. Besides standard histology, immunohistochemistry was performed using antibodies with markers for microglia, astrocytes, platelets, monocytes, and the proteins laminin, fibronectin, and collagen IV.
Results
Traces, layers, and plaques could be demonstrated in every investigated silicone or titanium valve with an implantation time of more than 6 days. Most of the tissue was found adjacent to silicone and titanium surfaces of the inner housing, the adjustment rotor, and ball-in-cone core. Markers for micro and astroglia stained positive in 40–60% of the specimen, mostly demonstrating a proteinous layer positive for laminin (80%), fibronectin (30%), and collagen IV (30%).
Conclusions
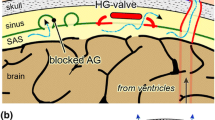
Tissue reactions with formation of cellular and proteinous matrix components are common in obstructed silicone and titanium shunt valves. The tissue mimics astrocytic repair mechanisms genuine for basilar membrane matrix. The knowledge of these typical arachnoid patterns of colonization is a prerequisite for developing future shunt devices.



Similar content being viewed by others
References
Adams KL, Gallo V (2017) The diversity and disparity of the glial scar. Nat Neurosci 21(1):9–15
Al-Maawi S, Orlowska A, Sader R, Kirkpatrick CJ, Ghanaati S (2017) In vivo cellular reactions to different biomaterials—physiological and pathological aspects and their consequences. Semin Immunol 29:49–61
Bock HC, Kanzler M, Thomale U-W, Ludwig H-C (2018) Implementing a digital real-time Hydrocephalus and Shunt Registry to evaluate contemporary pattern of care and surgical outcome in pediatric hydrocephalus. Childs Nerv Syst 34(3):457–464
Browd SR, Ragel BT, Gottfried ON, Kestle JRW (2006) Failure of cerebrospinal fluid shunts: part I: obstruction and mechanical failure. Pediatr Neurol 34(2):83–92
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol 11(4):318–326
Echizenya K, Satoh M, Mural H, Ueno H, Abe H, Komei T (1987) Mineralization and biodegradation of CSF shunting systems. J Neurosurg 67:584–591
Eymann R, Meier U, Kiefer M (2010) Animal experiments to evaluate complications of foreign materials on silicone with shunt catheters: preliminary results. Acta Neurochir Suppl 106(Chapter 15):91–93
Hao X, Junwen W, Jiaqing L, Ran L, Zhuo Z, Yimin H, Wei J, Wei S, Ting L (2016) High fibrosis indices in cerebrospinal fluid of patients with shunt-dependent post-traumatic chronic hydrocephalus. Transl Neurosci 7(1):92–97
Hao X, Zhangxiang W, Shaolin Z, Guowei T, Hongwei Z (2013) Procollagen type I C-terminal propeptide, procollagen type III N-terminal propeptide, hyaluronic acid, and laminin in the cerebrospinal fluid of rats with communicating hydrocephalus. J Neurosurg Pediatr 11(6):692–696
Harris CA, McAllister JP II (2012) What we should know about the cellular and tissue response causing catheter obstruction in the treatment of hydrocephalus. Neurosurgery 70(6):1589–1602
Mestre H, Hablitz LM, Xavier AL et al (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7:74
Morris AWJ, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO (2016) Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol 131(5):725–736
Nakada T, Kwee IL (2018) Fluid dynamics inside the brain carrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 45:107385841877502–107385841877512
Nakada T, Kwee I, Igarashi H, Suzuki Y (2017) Aquaporin-4 functionality and Virchow-Robin space water dynamics: physiological model for neurovascular coupling and glymphatic flow. Int J Mol Sci 18(8):1798–1714
NULSEN FE, SPITZ EB (1951) Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum 2:399–403
Plog BA, Nedergaard M (2018) The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13(1):379–394. https://doi.org/10.1146/annurev-pathol-051217-111018
Quentin T, Poppe A, Bär K, Sigler A, Foth R, Michel-Behnke I, Paul T, Sigler M (2009) A novel method for processing resin-embedded specimens with metal implants for immunohistochemical labelling. Acta Histochem 111(6):538–542
Quintana FJ (2017) Astrocytes to the rescue! Glia limitans astrocytic endfeet control CNS inflammation. J Clin Invest 127(8):2897–2899
Sigler M, Paul T, Grabitz RG (2005) Biocompatibility screening in cardiovascular implants. Z Kardiol 94(6):383–391
Sigler M, Klötzer J, Quentin T, Paul T, Möller O (2015) Stent implantation into the tracheo-bronchial system in rabbits: histopathologic sequelae in bare metal vs. drug-eluting stents. Mol Cell Pediatr 2(1):10
Soler GJ, Jaiswal D, Zaveri HP (2018) A review of cerebral shunts, current technologies, and future endeavors. Yale J Biol 91:313–321
Sridharan A, Rajan SD, Muthuswamy J (2013) Long-term changes in the material properties of brain tissue at the implant–tissue interface. J Neural Eng 10(6):066001
Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ (2015) Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Biochem Pharmacol 18(6):313–325
Suzuki K, Yamada K, Nakada K, Suzuki Y, Watanabe M, Kwee IL, Nakada T (2017) MRI characteristics of the glia limitans externa: a 7T study. Magn Reson Imaging 44:140–145
Tubbs RS, Muhleman M, Loukas M, Cohen-Gadol AA (2011) Ventriculoperitoneal shunt malfunction from cerebrospinal fluid eosinophilia in children: case-based update. Childs Nerv Syst 28(3):345–348
Whitelaw A (2001) Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol 6(2):135–146
Yabushita T (1995) Development of valve spring made of titanium alloy. JSAE Rev 16(1):112
Acknowledgments
We thank Mrs. Poppe and Mrs. Bär for excellent technical assistance preparing the specimen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval was obtained from the institutional ethic board no. 12/9/17.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ludwig, H., Reitemeyer, M., Bock, H. et al. Hydrocephalus shunt therapy: current titanium shunt valve implants obstructed by internal tissue proliferations identified as extracellular matrix membranes. Childs Nerv Syst 36, 2717–2724 (2020). https://doi.org/10.1007/s00381-019-04467-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04467-8