Abstract
Objects
The objectives were to present magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) findings in three patients with deletion on mitochondrial DNA (mtDNA) and nonclassical mitochondrial disorders (NCMD), correlating these findings with the percentage of deleted mtDNA.
Results
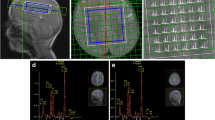
Our study confirms the high prevalence of white matter (WM), basal ganglia, and posterior fossa lesions in NCMD, ranging from mild to severe involvement. The subcortical WM, caudate, thalamus, globus pallidus, and dorsal brain stem were more frequently affected. A lactate peak was the most frequent finding at the MRS. We found a correlation between the percentage of mtDNA deletion and degree of MRS abnormalities.
Conclusions
Our findings showed that MRS is a useful investigational tool in patients with NCMD. Supplementary studies are necessary to elucidate the correlation of quantitative mtDNA deletion and neuroimaging phenotype.



Similar content being viewed by others
References
Singhal N, Gupta BS, Saigal R, Makkar J, Mathur R (2000) Mitochondrial diseases: an overview of genetics, pathogenesis, clinical features and an approach to diagnosis and treatment. J Postgrad Med 46:224–230
von Kleist-Retzow JC, Cormier-Daire V, Viot G et al (2003) Antenatal manifestations of mitochondrial respiratory chain deficiency. J Pediatr 143:208–212
Morgan-Hughes JA, Sweeney MG, Cooper JM et al (1995) Mitochondrial DNA (mtDNA) diseases: correlation of genotype to phenotype. Biochim Biophys Acta 1271:135–140
DiMauro S (2004) Mitochondrial diseases. Biochim Biophys Acta 1658:80–88
Bai RK, Wong LJ (2004) Detection and quantification of heteroplasmic mutant mitochondrial DNA by real-time amplification refractory mutation system quantitative PCR analysis: a single-step approach. Clin Chem 50:996–1001
von Kleist-Retzow JC, Schauseil-Zipf U, Michalk DV, Kunz WS (2003) Mitochondrial diseases—an expanding spectrum of disorders and affected genes. Exp Physiol 88:155–166
Park SB, Ma KT, Kook KH et al (2004) Kearns–Sayre syndrome—3 case reports and review of clinical feature. Yonsei Med J 45(4):727–735
Bianchi MC, Tosetti M, Battini R et al (2003) Proton MR spectroscopy of mitochondrial diseases: analysis of brain metabolic abnormalities and their possible diagnostic relevance. AJNR 24:1958–1966
Munoz A, Mateos F, Simon R, Garcia-Silva MT, Cabello S, Arenas J (1999) Mitochondrial diseases in children: neuroradiological and clinical features in 17 patients. Neuroradiology 41:920–928
Barragan-Campos HM, Vallee JN, Lo D et al (2005) Brain magnetic resonance imaging findings in patients with mitochondrial cytopathies. Arch Neurol 62(5):737–742
Sacher M, Fatterpekar GM, Edelstein S et al (2005) MRI findings in an atypical case of Kearns–Sayre syndrome: a case report. Neuroradiology 47(4):241–244
Leutner C, Layer G, Zierz S, Solymosi L, Dewes W, Reiser M (1994) Cerebral MR in ophthalmoplegia plus. AJNR 15:681–687
Kuwabara T, Watanabe H, Tanaka K et al (1994) Mitochondrial encephalomyopathy: elevated visual cortex lactate unresponsive to photic stimulation—a localized 1H-MRS study. Neurology 44:557–559
Ducreux D, Nasser G, Lacroix C et al (2005) MR diffusion tensor imaging, fiber tracking, and single-voxel spectroscopy findings in an unusual MELAS case. AJNR 26:1840–1844
Sparaco M, Bonilla E, DiMauro S, Powers JM (1993) Neuropathology of mitochondrial encephalomyopathies due to mitochondrial DNA defects. J Neuropathol Exp Neurol 52:1–10
Chabi B, Mousson de Camaret B, Duborjal H, Issartel JP, Stepien G (2003) Quantification of mitochondrial DNA deletion, depletion, and overreplication: application to diagnosis. Clin Chem 49:1309–1317
Ishikawa Y, Goto Y, Ishikawa Y, Minami R (2000) Progression in a case of Kearns–Sayre syndrome. J Child Neurol 15:750–755
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vedolin, L., Moura de Souza, C., Schwark, R. et al. Conventional MRI and MR spectroscopy in nonclassical mitochondrial disease: report of three patients with mitochondrial DNA deletion. Childs Nerv Syst 22, 1355–1359 (2006). https://doi.org/10.1007/s00381-006-0082-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-006-0082-y