Abstract
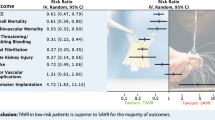
In aged population, the early and long-term outcomes of coronary revascularization (CABG) added to surgical aortic valve replacement (SAVR) compared to isolated SAVR (i-SAVR) are conflicting. To address this limitation, a meta-analysis comparing the early and late outcomes of SAVR plus CABG with i-SAVR was performed. Electronic databases from January 2000 to November 2021 were screened. Studies reporting early-term and long-term comparison between the two treatments in patients over 75 years were analyzed. The primary endpoints were in-hospital/30-day mortality and overall long-term survival. The pooled odd ratio (OR) and hazard ratio (HR) with 95% confidence interval (CI) were calculated for in-early outcome and long-term survival, respectively. Random-effect model was used in all analyses. Forty-four retrospective observational studies reporting on 74,560 patients (i-SAVR = 36,062; SAVR + CABG = 38,498) were included for comparison. The pooled analysis revealed that i-SAVR was significantly associated with lower rate of early mortality compared to SAVR plus CABG (OR = 0.70, 95% CI 0.66–0.75; p < 0.0001) and with lower incidence of postoperative acute renal failure (OR = 0.65; 95% CI 0.50–0.91; p = 0.02), need for dialysis (OR = 0.65; 95% CI 0.50–0.86; p = 0.002) and prolonged mechanical ventilation (OR = 0.57; 95% CI 0.42–0.77; p < 0.0001). Twenty-two studies reported data of long-term follow-up. No differences were reported between the two groups in long-term survival (HR = 0.95; 95% CI 0.87–1.03; p = 0.23). CABG added to SAVR is associated with worse early outcomes in terms of early mortality, postoperative acute renal failure, and prolonged mechanical ventilation. Long-term survival was comparable between the two treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The elderly population continues to increase in Europe and United States (US), especially age greater than 75 years. This group is expected to grow considerably over the next 20 years. In Europe, the population over 75 years is expected to reach 65 million by 2040, an approximately 49% increase compared to 2020 [1]. By 2040 in the United States, the population over age 75 is expected to rise from about 23 million today, to more than 43 million, a projected increase of about 90% [2, 3]. Because aortic valve stenosis (AS) and coronary artery disease (CAD) are the most commonly represented cardiac lesions in the elderly, as the elderly population increases a concomitant rise in AS and CAD is anticipated. Advanced age is associated with considerable number of comorbidities and medical frailty, exposing the elderly patient to potentially considerable operative risk. Moreover, simultaneous surgical aortic valve replacement (SAVR) and coronary artery bypass grafting (CABG) carries a higher procedural risk compared with isolated SAVR (i-SAVR). Indeed, even if AS could be addressed with transcatheter aortic valve implantation (TAVI) even in patients older than 75 years with intermediate or low risk [4], the combination of TAVI and percutaneous coronary intervention (PCI) is not a widely accepted practice, especially for those patients with a recognized heavily calcified CAD. Several studies [5,6,7,8,9,10] have reported relevant early results in patients who underwent either i-SAVR or SAVR combined with CABG. Other authors [11,12,13,14,15,16] have reported unfavorable early outcome in those patients who underwent simultaneous SAVR and CABG. The results are still debated regarding long-term outcomes, as some studies have reported acceptable and comparable long-term outcomes [17, 18], whereas other authors have reported conflicting results, with some studies showing better long-term survival in i-SAVR patients [19] and others reporting better long-term outcomes in SAVR plus CABG patients [20, 21]. No randomized control trials are available and, to the best of our knowledge, no meta-analyses have addressed the impact of concomitant CABG and SAVR in elderly patients. To address this limitation, a systematic review and meta-analysis was conducted with the best available evidence, evaluating the impact on early-term and long-term outcomes of CABG combined with SAVR, compared to i-SAVR in patients greater than 75 years of age.
Materials and methods
Systematic review of the literature, search strategy and eligibility criteria
A comprehensive review of previous relevant studies which were published from 1 January 2000 to 30 November 2021 was conducted. The search was conducted using the electronic databases PubMed and EMBASE. Search terms used alone or in combination included “elderly patients,” “very elderly,” “octogenarians,” “surgical aortic valve replacement,” “coronary artery bypass grafting,” “early-term results,” “75 years old” and “80 years old.” Furthermore, the references list of the obtained articles was used to implement the search.
The literature search and review were based on the PICOS format (Population; Intervention; Comparison; Outcomes; Studies); Population: patients with isolated aortic valve disease or combined with coronary artery disease; Intervention: i-SAVR; Comparison: SAVR plus CABG; Outcomes: early and long-term outcomes; Studies: randomized trials, retrospective and prospective observational studies.
Selection of relevant studies was conducted according to the following inclusion criteria: (1) patients who underwent either i-SAVR or SAVR in addition to CABG; (2) patients older than 75 years; (3) early mortality comparing the two surgical interventions; (4) long-term survival comparing the two operations; (5) studies included any of the following postoperative complications: atrial fibrillation (POAF), acute renal failure, need for dialysis, pneumonia, prolonged mechanical ventilation (PMV), stroke, re-thoracotomy for bleeding/tamponade, need for postoperative intra-aortic balloon pump (IABP), length of stay and early mortality. Studies including in the analysis other associated cardiac procedure were excluded. Moreover, studies which were published in languages other than English were excluded, as were commentaries, letters, case reports, systematic reviews and meta-analyses.
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22] and was based on the following steps: (1) identification of titles and abstracts of records through database search; (2) removal of duplicates; (3) screening and selection of abstracts; (4) evaluation of study eligibility through full-text articles; and (5) final inclusion in study. Studies were selected by two independent authors (SDA, DT). When there was disagreement, a third senior author (FF) reviewer made the decision of whether to include or exclude the study.
The study protocol of the systematic review and meta-analysis was registered and published online in PROSPERO (The International Prospective Register of Systematic Reviews; ID: CRD42021276831).
Data extraction and database
Two reviewers (SDA and DT) independently performed data extraction which were reported in a standard table sheet database (Microsoft Office Excel 2016, Microsoft, Redmond, WA, USA). Median and interquartile ranges were converted into mean and standard deviations following the recommendations of Luo et al. [23]. All studies included in the meta-analysis were identified by first author, country, study design, study period, and year of publication. The following patient factors were collected: age, gender (male), POAF, postoperative acute renal failure, need for dialysis, postoperative pneumonia, PMV, postoperative stroke, re-thoracotomy for bleeding/tamponade, postoperative IABP, postoperative length of stay.
Endpoints
The primary endpoints of the meta-analysis were the (i) early mortality, defined as death occurred within 30 days or during the index admission and (ii) the overall long-term survival. The secondary endpoints were the following postoperative complications: new onset of POAF, renal failure, need for dialysis, pneumonia, PMV (> 48 h), any stroke, re-thoracotomy for bleeding, need for IABP and length of stay.
Statistical analysis
The pooled effect size with odd ratio (OR) and 95% Confidence Interval (CI) using the Mantzel–Haenszel method was calculated for early mortality and for the secondary endpoints. The pooled hazard ratio (HR) with 95% CI using the Mantzel–Haenszel method was calculated for long-term survival. The random-effect model was preferred because the variability across the studies was taken into account in the model. HR and the corresponding 95% CI was calculated analysing time-to event outcomes according to the methods proposed by Tierney et al. [24]. When available, the reported HRs of selected studies were compared with the estimated HRs.
Weighted mean differences were calculated for the continuous variable length of stay. Forest plots were created to represent the primary and secondary outcomes and to determine the effect size. Statistical heterogeneity was assessed with Chi-square test and I2 test and defined as low for I2 ranging from 0% to 25%, moderate for I2 ranging from 26% to 50% and high for I2 above 50% [25]. Publication bias was assessed for each endpoint by generating the funnel plots using the trim and fill method and analysed by means of Egger’s test and Begg and Mazumdar’s test and estimated visually. Possible publication bias was suggested also by asymmetric funnel plot. Sensitivity analysis was applied to verify the influence of a single study on the primary endpoints, by sequentially removing one study, according to the leave-one-out method [26].
Categorical variables were reported as number and percentages. Continuous variables were reported as mean and standard deviation. A two-tailed p-value < 0.05 was considered to indicate statistical significance. All statistical analyses were completed with ProMeta3 software (http://idostatistics.com/prometa3/), and with the Review Manager (RevMan5) Version 5.3 (The Cochrane Collaboration, 2012, The Nordic Cochrane Centre and Copenhagen, Denmark).
Results
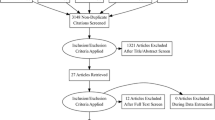
A total of 2046 titles and abstracts were identified, of which 57 were considered potentially relevant and for the meta-analysis and retrieved as full-text. After evaluating the full-text articles, 44 studies [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] fulfilled the eligibility criteria and were included in the final analysis. All included studies were observational and retrospective in design, an no randomized clinical trials or prospective studies were identified. The PRISMA Flow Chart of study selection process is shown in Supplemental Fig. 1.
A total of 74,560 patients were extracted from the selected articles. I-SAVR included 36,062 patients (48.5%) and SAVR plus CABG included 38,498 patients (51.5%). Characteristics of studies, and preoperative data of patients included in each study are shown in Table 1. Postoperative data are listed in Table 2.
Primary endpoints: early mortality and long-term survival
All studies included in the meta-analysis reported the early mortality comparison between i-SAVR and SAVR combined with CABG. The pooled analysis revealed a significant difference between the two groups, favoring i-SAVR treatment (OR 0.70, 95% CI 0.66–0.75; p < 0.0001) with no evidence of heterogeneity (I2 = 0%, Tau2 = 0.01, p = 0.69) (Fig. 1). The leave-one-out analysis did not identify any influential studies on the aggregated data, with each study removed each time and the meta-analysis repeated n times the number of studies included in the analysis. (Supplemental Fig. 2A). Funnel plot analysis did not reveal asymmetry around the axis, with no evidence of publication bias (Egger’s linear regression test: p = 0.06; Begg and Mazumdar’s test: p = 0.15) (Supplemental Fig. 3A).
Twenty-two studies [5, 7, 9, 11, 13, 17,18,19,20,21, 27, 29, 34, 38, 39, 41, 45, 50,51,52,53] reported long-term survival comparison between the two surgical interventions with a mean follow-up ranging from 2.1 years [27] to 9.5 years [45]. The weighted mean follow-up was 3.2 years. The longest follow-up was 16.1 years [10]. The pooled analysis of long-term survival did not reveal difference between the two treatments (HR = 0.95; 95% CI 0.87–1.03; p = 0.23) with evidence of low heterogeneity (I2 = 16%; Tau2 = 0.01; p = 0.24) (Fig. 2A).
A. Forest plot of comparison for long-term survival. B. Forest plot of comparison for long-term survival of studies reporting a maximum follow-up of 10 years or more. C. Forest plot for new onset of postoperative atrial fibrillation. I-SAVR isolated surgical aortic valve replacement; CABG coronary artery bypass grafting; M–H Mantzel–Haenszel
The leave-one-out analysis did not identify any influential studies on the pooled data. (Supplemental material, Fig. 2B). No evidence of publication bias was found assessed by the Egger’s linear regression test (p = 0.27) and Begg and Mazumdar’s linear regression test (p = 0.19) or by visual inspection of the funnel plot (Supplemental material, Fig. 3B). When we restricted the analysis for those studies (n = 12) [7, 9, 10, 13, 19, 20, 38, 39, 41, 45, 51, 52] reporting a maximum follow-up of 10 years or more, the pooled analysis revealed no differences between the two groups (HR = 0.97; 95% CI 0.87–1.09; p = 0.64) with evidence of moderate heterogeneity (I2 = 44%; Tau2 = 0.01; p = 0.05) (Fig. 2B) and with no evidence of publication bias (Egger’s linear regression test: p = 0.83; Begg and Mazumdar’s test: p = 0.90) (Supplemental material, Fig. 3C). The weighted mean follow-up and was 5.3 years.
Secondary endpoints
The odds of postoperative atrial fibrillation were comparable between the two groups (OR = 0.83; 95% CI 0.64 –1.07; p = 0.15) with significant heterogeneity (I2 = 62%) (Fig. 2C). Postoperative acute renal failure incidence was reduced in patients received i-SAVR compared to those received additional CABG (OR = 0.60; 95% CI 0.40–0.91; p = 0.02), with evidence of moderate heterogeneity (I2 = 37%) (Fig. 3A). Similarly, reduced odds of postoperative dialysis were more represented in patients who underwent i-SAVR compared to SAVR + CABG (OR = 0.66; 95% CI 0.50–0.86; p = 0.002), with no evidence of heterogeneity (I2 = 0%) (Fig. 3B). I-SAVR group had a nonsignificant reduced odds of postoperative IABP usage (OR = 0.55; 95% CI 0.30–1.04; p = 0.07), with no heterogeneity (I2 = 0%) (Fig. 3C). No significant differences were found regarding length of postoperative hospital stay (mean difference = −0.57; 95% CI −1.35–0.22; p = 0.16; I2 = 0%) (Fig. 4A). Reduced odds of PMV were observed in patients who underwent i-SAVR (OR = 0.67; 95% CI 0.40–1.12; p < 0.13) with high heterogeneity (I2 = 67%) (Fig. 4B). No differences between the two operations were observed regarding re-thoracotomy for bleeding/tamponade (OR = 0.89; 95% CI 0.62–1.26; p = 0.50; I2 = 40%) (Fig. 4C), postoperative stroke (OR = 0.91; 95% CI 0.66–1.25; p = 0.56; I2 = 0%) (Fig. 5A) and postoperative pneumonia (OR = 0.73; 95% CI 0.40–1.32; I2 = 0%) (Fig. 5B). The pooled effect sizes are summarized in the Fig. 6. Analysis of the funnel plots showed symmetry and no evidence of risk of publication bias (Supplemental Materials, Fig. 3D through 3N).
Discussion
AS is the most frequently identified lesion in the elderly patients, with incidence increasing with age, exceeding 5% in patients over 80 years [54, 55]. Previous studies reported that almost half of the elderly patients undergoing SAVR were more likely to require CABG, compared to the non-elderly requiring SAVR [56,57,58].
By this comprehensive systematic review and meta-analysis, we aimed to analyze the impact of CABG in the aged population requiring SAVR, and to the best of our knowledge this is the first meta-analysis focusing on this topic. The main findings where that (i) CABG in combination with SAVR is associated with higher early mortality compare to i-SAVR, (ii) the long-term survival is comparable between the two surgical operations and (iii) CABG plus SAVR is associated with a higher rate of postoperative complications such as acute renal failure, need for dialysis, and PMV. Interestingly, the rate of new onset POAF, IABP usage, postoperative stroke, re-thoracotomy for bleeding/tamponade, postoperative pneumonia and length of hospital stay were similar in both groups.
CAD has an unfavorable prognostic factor, accentuated even further in presence of left main stenosis greater than 50%. Such patients have an increased risk of developing myocardial injury likely secondary to an imbalance between myocardial oxygen supply and demand during cardiac surgery. Previous studies demonstrated that cardiac troponin (c-Tn) levels measured after cardiac surgery predict early mortality [59]. C-Tn levels and mortality increase with increasing complexity of cardiac surgery, such that the median c-Tn level rises progressively in patients undergoing isolated CABG with a single graft compared with 2 or more grafts [60].
Increased duration of cardiopulmonary bypass (CPB) and aortic cross clamping (X-Clamp) times in the elderly remain a concern. Longer CPB time is associated with increased incidence of cerebral, renal and coagulopathy, and greater X-Clamp time induces increased risk of myocardial damage, due to the lower efficiency of the physiological pathways of homeostasis. Furthermore, patients with severe CAD are more frequently affected by peripheral artery disease, which can increase the risk of postoperative ischemic complications with unfavorable outcomes, especially in elderly patients. A heart team approach including surgeons, cardiologists, anesthesiologists, internist and physiotherapists can be helpful to assess these elderly candidates and choose the best approach to treat AS [4]. For high-risk candidates, minimally invasive treatment options are desirable. Over the last decade, transcatheter aortic valve implantation (TAVI) has been identified as the standard of care for high-surgical risk patients, or for those considered inoperable by cardiac surgeons. The switch from SAVR to TAVI for elderly patients during recent years has led to a significant decrease of early mortality following AVR [61]. TAVI has demonstrated the potential to decrease the morbidity associated with standard SAVR owing to the avoidance of a median sternotomy, cardiopulmonary bypass and cardioplegic arrest.
Data from the recent randomized SURTAVI trial, comparing TAVI with PCI versus SAVR plus CABG in 332 patients, reported a 30-day mortality of 4.1% vs 3.7%, respectively, an incidence of stroke of 3.6% vs 4.3% and advanced acute kidney injury of 1.8% vs 3.7% [62]. The study excluded patients with SYNTAX score > 22, however, therefore it is not possible to extrapolate the outcomes from patients with more advanced CAD. Noteworthily the current guidelines for CAD recommend CABG for a high SYNTAX score and these patients could benefit from SAVR with CABG [63].
The German Aortic Valve Registry, an all-comers registry including 85 German centers, recently showed that the rate of in-hospital mortality for 26,618 patients undergoing isolated SAVR was 1.7%. The 30-day mortality in the 16,158 patients who underwent SAVR and CABG was significantly higher at 3.3%. In the SAVR plus CABG cohort stratified according to STS score risk, in 4044 patients in the intermediate category (STS score 4–8%), the in-hospital rate of mortality was 5.4%, the rate of disabling stroke was 2.4%, and need for new pacemaker or implantable cardioverter-defibrillator was 4.6% [64].
No unfavorable impact of CABG in combination with SAVR on long-term mortality compared with i-SAVR was reported in this updated systematic review and meta-analysis. The comparable long-term survival between the two treatments may support the rationale that CAD, although a recognized additional risk factor, when associated with aortic valve disease, probably does not result in increased long-term mortality when addressed with CABG. Among the 23 studies that reported follow-up data, there was a high range of mean follow-up times, varying from 2.1 to 6.5 years. Interestingly, when we narrowed the analysis to those studies that reported a maximum follow-up time of 10 years or more, again no differences were reported between the two treatments. Some authors have observed a long-term benefit of patients with concomitant CAD and AS undergoing CABG plus SAVR compared with patients who did not receive the CABG procedure at the time of SAVR [65]. The relief of AS, along with the addition of coronary revascularization, would increase coronary flow reserve and provide reversal remodeling as in patients with isolated AS who underwent i-SAVR. These factors would promote regression of left ventricular hypertrophy and increased coronary microcirculation, which are critical determinants of long-term survival [66].
In the meta-analysis, it is interesting to emphasize the validity and safety of the conventional surgical approach in elderly patients. Once the patient has gone through the postoperative period, where the CBAG + SAVR combination is associated with higher hospital mortality, long-term survival remains comparable between the two treatments. This finding has its clinical relevance and allows confirmation of the validity and safety of the conventional surgical approach, as well as that the associated CBAG has no negative clinical impact in the long term.
The incidence of new onset POAF increases with advancing age and the multifactorial pathophysiology has not been completely elucidated [67]. In this meta-analysis, no significant difference in postoperative AF incidence was identified between the two populations. One possible explanation for these data could be the higher incidence of POAF in elderly patients, regardless of the type of cardiac surgical procedure to which they undergo. In addition, severe aortic valve stenosis is a chronic disease that can lead to remodeling of the left ventricle with a decrease in diastolic compliance leading to increased left atrial volume and altered atrial function. Although CAD increases the risk of developing POAF [67], in this study, CABG was not associated with the development of POAF.
As age is an established risk factor for atherosclerotic disease, so there is an increased risk of aortic calcifications is expected in elderly patients [68]. Postoperative acute renal failure and dialysis appear to be lower in i-SAVR compared to SAVR plus CABG. A possible explanation is the increased rate of diabetes, hypertension, vascular disease, preoperative renal failure which are more represented in patients with CAD and longer CPB time in patients who underwent SAVR + CABG compared to i-SAVR [69,70,71]. CABG added to SAVR shows a nonsignificant trend toward a greater need for postoperative IABP compared to i-SAVR. Longer CPB and aortic X-clamp times, prolonged operative time, and peripheral vascular disease are predictive for postoperative IABP [72, 73]. These factors may explain why patients who underwent CABG in combination with SAVR had a higher incidence of postoperative IABP implantation. The meta-analysis shows that PMV was significantly associated with the SAVR + CABG surgical operation. Longer CPB time is reported to be an independent predictor of postoperative respiratory failure [74] and PMV (> 24 h) [75]. Since SAVR + CABG operation has a CPB time longer than i-SAVR, we can argue that this factor might be determinant in increasing the incidence of postoperative PMV in patients who received CABG added to SAVR. From the 17 studies that reported incidence of postoperative stroke, no significant differences emerged in patients undergoing i-SAVR compared to SAVR plus CABG. A plausible explanation for this finding is the pathophysiology of ischemic stroke post cardiac surgery. In patients undergoing aortic valve surgery, thromboembolism is likely attributable to aortic clamping and manipulation, as well as aortic valve decalcification, rather than to the duration of surgery [76, 77]. As cardiopulmonary bypass is required for both i-SAVR and CABG plus SAVR, similar thromboembolism rates would expect, since that both operations share the aortic manipulation.
Limitations
The meta-analysis shares the limitation of meta-analyses of retrospective observational studies that can be affected from a risk of treatment allocation bias and unmeasured confounders. Moreover, the results of some studies included in the analysis are limited by a relatively small numbers of patients. In addition, it was not possible to extrapolate the incidence of incomplete myocardial revascularization data of those patients affected by aortic valve disease and CAD who were treated with only i-SAVR. In such a scenario, it is not possible to analyze the impact of untreated CAD in SAVR. Moreover, data related to survival outcome were not reported by each study included in the meta-analysis and therefore the reported pooled data on long-term survival needs to be interpreted with a word of caution. Finally, it was not possible to extrapolate patient selection criteria towards either conventional surgery or TAVI, and, therefore these results may be influenced by selection bias, as the elderly patients included in each study were likely fit for surgery. However, the large number of patients included in the meta-analysis may reduce the aforementioned bias and allows for robust results.
Conclusions
In conclusion, in a meta-analysis of retrospective observational studies comparing early and long-term outcomes of patients undergoing aortic valve surgery, CABG in combination with SAVR is associated with a significantly higher incidence of 30-day mortality, whereas in the long-term follow-up the two treatments are comparable. Among the analyzed postoperative complications, CABG in combination with SAVR is associated with a higher incidence of acute renal failure, need for dialysis and PMV compared with i-SAVR. The incidence of postoperative stroke, POAF, need for IABP, re-thoracotomy for postoperative bleeding/tamponade, and length of stay were similar between the two treatments.
Change history
04 September 2022
Missing Open Access funding information has been added in the Funding Note.
References
https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_pjan&lang=en (21/11/2021)
https://www.jchs.harvard.edu/sites/default/files/jchs housing_americas_older_adults_2014-ch2_0.pdf. (21/11/2021)
Ortaman JM, Velkoff VA, Hogan H (2021) An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html. 21/11/2021
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, ESC/EACTS Scientific Document Group; ESC Scientific Document Group (2021) ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 60:727–800
Nikolaidis N, Pousios D, Haw MP, Kaarne M, Barlow CW, Livesey SA, Tsang GM, Ohri SK (2011) Long-term outcomes in octogenarians following aortic valve replacement. J Card Surg 26:466–471
Litmathe J, Boeken U, Feindt P, Gams E (2003) Concomitant CABG-procedures in elderly patients undergoing aortic valve replacement. An additional risk factor? Z Kardiol 92:947–952
Roberts WC, Ko JM, Filardo G, Kitchens BL, Henry AC, Hebeler RF Jr, Cheung EH, Matter GJ, Hamman BL (2007) Valve structure and survival in quadragenarians having aortic valve replacement for aortic stenosis (+/-aortic regurgitation) with versus without coronary artery bypass grafting at a single US medical center (1993 to 2005). Am J Cardiol 100:1683–1690
Harris RS, Yan TD, Black D, Black D, Bannon PG, Bayfield MS, Hendel PN, Wilson MK, Vallely MP (2013) Outcomes of surgical aortic valve replacement in octogenarians. Heart Lung Circ 22:618–626
Kuo K, Shah P, Hiebert B, Love K, Menkis AH, Manji RA, Arora RC (2017) Predictors of survival, functional survival, and hospital readmission in octogenarians after surgical aortic valve replacement. J Thorac Cardiovasc Surg 154:1544-1553.e1
Formica F, Mariani S, D’Alessandro S, Singh G, Di Mauro M, Cerrito MG, Messina LA, Scianna S, Papesso F, Sangalli F (2020) Does additional coronary artery bypass grafting to aortic valve replacement in elderly patients affect the early and long-term outcome? Heart Vessels 35:487–501
Folkmann S, Gorlitzer M, Weiss G, Harrer M, Thalmann M, Poslussny P, Grabenwoger M (2010) Quality of life in octogenarians one year after aortic valve replacement with or without coronary artery bypass surgery. Interact Cardiovasc Thorac Surg 11:750–753
Kolh P, Kerzmann A, Honore C, Comte L, Limet R (2007) Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur J Cardiothorac Surg 31:600–606
Likosky DS, Sorensen MJ, Dacey LJ, Baribeau YR, Leavitt BJ, Di Scipio AW, Hernandez Jr F, Cochran RP, Quinn R, Helm RE, Charlesworth DC, Clough RA, Malenka DJ, Sisto DA, Sardella G, Olmstead EM, Ross CS, O’Connor GT, Northern New England Cardiovascular Disease Study Group (2009) Long-term survival of the very elderly undergoing aortic valve surgery. Circulation 120(11 Suppl):S127–S133
Langanay T, Flécher E, Fouquet O, Ruggieri VG, De La Tour B, Félix C, Lelong B, Verhoye JP, Corbineau H, Leguerrier A (2012) Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 93:70–77
Agarwal S, Garg A, Parashar A, Svensson LG, Tuzcu EM, Navia JL, Mick S, Kapadia SR (2015) In-hospital mortality and stroke after surgical aortic valve replacement: a nationwide perspective. J Thorac Cardiovasc Surg 150:571–8.e8
Dimagli A, Sinha S, Caputo M, Angelini GD, Benedetto U (2020) Trend in morbidity and mortality in surgical aortic valve re-placement: a retrospective, observational, single centre study. Interact Cardiovasc Thorac Surg 31:796–802
Chiappini B, Camurri N, Loforte A, Di Marco L, Di Bartolomeo R, Marinelli G (2004) Outcome after aortic valve replacement in octogenarians. Ann Thorac Surg 78:85–89
Dell’Amore A, Aquino TM, Pagliaro M, Lamarra M, Zussa C (2012) Aortic valve replacement with and without combined coronary bypass grafts in very elderly patients: early and long-term results. Eur J Cardiothorac Surg 41:491–498
Krane M, Voss B, Hiebinger A, Deutsch MA, Wottke M, Hapfelmeier A, Badiu CC, Bauernschmitt R, Lange R (2011) Twenty years of cardiac surgery in patients aged 80 years and older: risks and benefits. Ann Thorac Surg 91:506–513
Melby SJ, Zierer A, Kaiser SP, Guthrie TJ, Keune JD, Schuessler RB, Pasque MK, Lawton JS, Moazami N, Moon MR, Damiano RJ Jr (2007) Aortic valve replacement in octogenarians: risk factors for early and late mortality. Ann Thorac Surg 83:1651–1656
Grau JB, Mak AW, Ferrari G, Johnson CK, Shaw RE, Sperling J, Brizzio ME, Zapolanski A (2014) Perioperative predictors of mid-term survival after aortic valve replacement. Asian Cardiovasc Thorac Ann 22:566–573
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group. Preferred reporting items for systematic reviews and me-ta-analyses: the PRISMA statement. Open Med 3:e123–e130
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27:1785–1805
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Viechtbauer W, Cheung MW (2010) Outlier and influence diagnostics for meta-analysis. Res Synth Methods 1:112–125
Thulin LI, Sjogren JL (2000) Aortic valve replacement with and without concomitant coronary artery bypass surgery in the elderly: risk factors related to long-term survival. Croat Med J 41:406–409
Ennker J, Mortasawi A, Gehle S, Yaghmaie M, Schröder T, Rosendahl U, Ennker IC (2001) Aortic valve replacement with or with-out concomitant coronary artery bypass grafting in the ninth decade of life. Langenbecks Arch Surg 386:272–277
Brunvand H, Offstad J, Nitter-Hauge S, Svennevig JL (2002) Coronary artery bypass grafting combined with aortic valve replacement in healthy octogenarians does not increase postoperative risk. Scand Cardiovasc J 36:297–301
Lam BK, Hendry PJ (2004) Patients over 80 years: quality of life after aortic valve replacement. Age Ageing 33:307–309
Unic D, Leacche M, Paul S, Rawn JD, Aranki SF, Couper GS, Mihaljevic T, Rizzo RJ, Cohn LH, O’Gara PT, Byrne JG (2005) Early and late results of isolated and combined heart valve surgery in patients > or =80 years of age. Am J Cardiol 95:1500–1503
Urso S, Sadaba R, Greco E, Pulitani I, Alvarez L, Juaristi A, Goiti JJ (2007) One-hundred aortic valve replacements in octogenarians: outcomes and risk factors for early mortality. J Heart Valve Dis 16:139–144
Bose AK, Aitchison JD, Dark JH (2007) Aortic valve replacement in octogenarians. J Cardiothorac Surg 2:33
Huber CH, Goeber V, Berdat P, Carrel T, Eckstein F (2007) Benefits of cardiac surgery in octogenarians—a postoperative quality of life assessment. Eur J Cardiothorac Surg 31:1099–1105
Ngaage DL, Cowen ME, Griffin S, Guvendik L, Cale AR (2008) Early neurological complications after coronary artery bypass grafting and valve surgery in octogenarians. Eur J Cardiothorac Surg 33:653–659
Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Silvay G, Adams DH (2008) Excellent early and late outcomes of aortic valve replacement in people aged 80 and older. J Am Geriatr Soc 56:255–261
Maillet JM, Somme D, Hennel E, Lessana A, Saint-Jean O, Brodaty D (2009) Frailty after aortic valve replacement (AVR) in octogenarians. Arch Gerontol Geriatr 48:391–396
Florath I, Albert A, Boening A, Ennker IC, Ennker J (2010) Aortic valve replacement in octogenarians: identification of high-risk patients. Eur J Cardiothorac Surg 37:1304–1310
Maslow A, Casey P, Poppas A, Schwartz C, Singh A (2010) Aortic valve replacement with or without coronary artery bypass graft surgery: the risk of surgery in patients > or =80 years old. J Cardiothorac Vasc Anesth 24:18–24
Yamane K, Hirose H, Youdelman BA, Bogar LJ, Diehl JT (2011) Conventional aortic valve replacement for elderly patients in the current era. Circ J 75:2692–2698
Kesavan S, Iqbal A, Khan Y, Hutter J, Pike K, Rogers C, Turner M, Townsend M, Baumbach A (2011) Risk profile and outcomes of aortic valve replacement in octogenarians. World J Cardiol 3:359–366
Raja SG, Navaratnarajah M, Husain M, Walker CP, Ilsley CD, Bahrami TT, Gaer JA, Amrani M (2013) Impact of concomitant coronary artery bypass grafting on in-hospital outcome in octogenarians undergoing aortic valve replacement. J Heart Valve Dis 22:177–183
Abel NJ, Rogal GJ, Burns P, Saunders CR, Chamberlain RS (2013) Aortic valve replacement with and without coronary artery by-pass graft surgery in octogenarians: is it safe and feasible? Cardiology 124:163–173
Mitchell AE, Mitchell IM (2013) The hidden risks of advancing age and concomitant ischemic heart disease after aortic valve re-placement. Clin Cardiol 36:129–132
Sasaki Y, Hirai H, Hosono M, Bito Y, Nakahira A, Suehiro Y, Kaku D, Okada Y, Suehiro S (2013) Adding coronary artery bypass grafting to aortic valve replacement increases operative mortality for elderly (70 years and older) patients with aortic steno-sis. Gen Thorac Cardiovasc Surg 61:626–631
Davis JP, LaPar DJ, Crosby IK, Kern JA, Lau CL, Kron IL, Ailawadi G (2014) Nonagenarians undergoing cardiac surgery. J Card Surg 29:600–604
Ho E, Mathur MN, Brady PW, Marshman D, Brereton RJ, Ross DE, Bhindi R, Hansen PS (2014) Surgical aortic valve replacement in very elderly patients aged 80 years and over: evaluation of early clinical outcomes. Heart Lung Circ 23:242–248
Kamiya H, Tanzeem N, Akhyari P, Pedraza A, Kallenbach K, Lichtenberg A, Karck M (2014) Impact of severe postoperative complications after cardiac surgery on mortality in patients aged over 80 years. Ann Thorac Cardiovasc Surg 20:383–389
Budniak W, Buczkowski P, Perek B, Katyńska I, Jemielity M (2014) Early and long-term results of cardiosurgical treatment of coronary artery disease and aortic stenosis in patients over 80 years old. Kardiochir Torakochirurgia Pol 11:246–251
Salsano A, Regesta T, Viganò G, Rapetto F, Boeddu S, Sportelli E, Pansini S, Risso P, Onorati F, Passerone G, Santini F (2016) Expectation and quality of life after aortic valve replacement over 85 years of age match those of the contemporary general population. Int J Artif Organs 39:56–62
Wang TK, Choi DH, Ramanathan T, Ruygrok PN (2017) Aortic valve replacement with or without concurrent coronary artery bypass grafting in octogenarians: eight-year cohort study. Heart Lung Circ 26:82–87
Ennker J, Zadeh B, Pons-Kuehnemann J, Niemann B, Grieshaber P, Ennker IC, Boening A (2018) Stentless bioprostheses for aortic valve replacement in octogenarians: the influence of coronary artery disease. Thorac Cardiovasc Surg 66:322–327
Takagi K, Arinaga K, Takaseya T, Otsuka H, Shojima T, Shintani Y, Zaima Y, Saku K, Oryoji A, Hiromatsu S (2020) Aortic valve replacement with or without concomitant coronary artery bypass grafting in very elderly patients aged 85 years and older. Heart Vessels 35:1409–1418
Carabello BA, Paulus WJ (2009) Aortic stenosis. Lancet 373:956–966
Iung B, Vahanian A (2011) Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 8:162–172
Barreto-Filho JA, Wang Y, Dodson JA, Desai MM, Sugeng L, Geirsson A, Krumholz HM (2013) Trends in aortic valve replacement for elderly patients in the United States, 1999–2011. JAMA 310:2078–2085
Vasques F, Lucenteforte E, Paone R, Mugelli A, Biancari F (2012) Outcome of patients aged >80 years undergoing combined aortic valve replacement and coronary artery bypass grafting: a systematic review and meta-analysis of 40 studies. Am Heart J 164:410-418.e1
Ashikhmina EA, Schaff HV, Dearani JA, Sundt TM 3rd, Suri RM, Park SJ, Burkhart HM, Li Z, Daly RC (2011) Aortic valve re-placement in the elderly: determinants of late outcome. Circulation 124:1070–1078
Croal BL, Hillis GS, Gibson PH, Fazal MT, El-Shafei H, Gibson G, Jeffrey RR, Buchan KG, West D, Cuthbertson BH (2006) Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 114:1468–1475
Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, Engoren M, Alexander JH, Levy JH, Chaitman BR, Broderick S, Mack MJ, Pieper KS, Farkouh ME (2011) Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 305:585–591
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364:2187–2198
Sondergaard L, Popma JJ, Reardon MJ, Van Mieghem NM, Deeb GM, Kodali S, George I, Williams MR, Yakubov SJ, Kappetein AP, Serruys PW, Grube E, Schiltgen MB, Chang Y, Engstrøm T, SURTAVI Trial Investigators (2019) Comparison of a complete percutaneous versus surgical approach to aortic valve replacement and revascularization in patients at intermediate surgical risk: results from the randomized SURTAVI trial. Circulation 140:1296–1305
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group (2019) ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 40:87–165
Fujita B, Ensminger S, Bauer T, Möllmann H, Beckmann A, Bekeredjian R, Bleiziffer S, Schäfer E, Hamm CW, Mohr FW, Katus HA, Harringer W, Walther T, Frerker C, GARY Executive Board (2018) Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: an update from the German Aortic Valve Registry on 42 776 patients. Eur J Cardio-thorac Surg 53:552–559
Thalji NM, Suri RM, Daly RC, Greason KL, Dearani JA, Stulak JM, Joyce LD, Burkhart HM, Pochettino A, Li Z, Frye RL, Schaff HV (2015) The prognostic impact of concomitant coronary artery bypass grafting during aortic valve surgery: implications for revascularization in the transcatheter era. J Thorac Cardiovasc Surg 149:451–460
Patel KP, Michail M, Treibel TA, Rathod K, Jones DA, Ozkor M, Kennon S, Forrest JK, Mathur A, Mullen MJ, Lansky A, Baumbach A (2021) Coronary revascularization in patients undergoing aortic valve replacement for severe aortic stenosis. JACC Cardiovasc Interv 14:2083–2096
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group (2021) 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42:373–498
Stone K, Fryer S, Faulkner J, Meyer ML, Heffernan K, Kucharska-Newton A, Zieff G, Paterson C, Matsushita K, Hughes TM, Tanaka H, Stoner L (2022) Associations of lower-limb atherosclerosis and arteriosclerosis with cardiovascular risk factors and disease in older adults: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis 340:53–60
Axtell AL, Fiedler AG, Melnitchouk S, D’Alessandro DA, Villavicencio MA, Jassar AS, Sundt TM 3rd (2020) Correlation of cardiopulmonary bypass duration with acute renal failure after cardiac surgery. J Thorac Cardiovasc Surg 159:170–178
Brown JR, Kramer RS, MacKenzie TA, Coca SG, Sint K, Parikh CR (2012) Determinants of acute kidney injury duration after cardiac surgery: an externally validated tool. Ann Thorac Surg 93:570–576
Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M (2012) Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 26:64–69
Ergüneş K, Yurekli I, Celik E, Yetkin U, Yilik L, Gurbuz A (2013) Predictors of intra-aortic balloon pump insertion in coronary surgery and mid-term results. Korean J Thorac Cardiovasc Surg 46:444–448
Parissis H, Leotsinidis M, Akbar MT, Apostolakis E, Dougenis D (2010) The need for intra-aortic balloon pump support following open heart surgery: risk analysis and outcome. J Cardiothorac Surg 5:20
Zainab A, Nguyen DT, Graviss EA, Fatima S, Masud FN, MacGillivray TE (2022) Development and validation of a risk score for respiratory failure after cardiac surgery. Ann Thorac Surg 113:577–584
Aksoy R, Karakoc AZ, Cevirme D, Elibol A, Yigit F, Yilmaz Ü, Rabus MB (2021) Predictive factors of prolonged ventilation following cardiac surgery with cardiopulmonary bypass. Braz J Cardiovasc Surg 36:780–787
Sultan I, Bianco V, Kilic A, Jovin T, Jadhav A, Jankowitz B, Aranda-Michel E, D’angelo MP, Navid F, Wang Y, Thoma F, Gleason TG (2020) Predictors and outcomes of ischemic stroke after cardiac surgery. Ann Thorac Surg 110:448–456
Butler CG, Ho Luxford JM, Huang CC, Ejiofor JI, Rawn JD, Wilusz K, Fox JA, Shernan SK, Muehlschlegel JD (2017) Aortic atheroma increases the risk of long-term mortality in 20,000 patients. Ann Thorac Surg 104:1325–1331
Acknowledgements
The authors would like to thank Giorgia Pavan for her tireless assistance and valuable contribution to the preparation, writing and editing of the article.
Funding
This study has not received funding. Open access funding provided by Università degli Studi di Parma within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Alessandro, S., Tuttolomondo, D., Singh, G. et al. The early and long-term outcomes of coronary artery bypass grafting added to aortic valve replacement compared to isolated aortic valve replacement in elderly patients: a systematic review and meta-analysis. Heart Vessels 37, 1647–1661 (2022). https://doi.org/10.1007/s00380-022-02073-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02073-4