Abstract
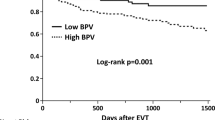
We sought to investigate the effect of ward-to-cath lab blood pressure (BP) differences on long-term clinical outcomes in patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stent (DES). There are limited data available on the association between PCI with DES and BP differences on long-term clinical outcomes. This study enrolled 994 patients who underwent PCI with DES from March 2003 to August 2007. Resting BP was measured in a ward environment before transfer to the cardiac catheterization lab (cath lab), and again when the patient was laid down on the cath lab table. Patients were divided into two groups according to the difference in ward-to-cath lab systolic BP. Large difference group (n = 383) was defined as the absolute systolic difference of >20 mmHg and small difference group (n = 424) as the absolute systolic difference of ≤20 mmHg. The primary endpoints were all-cause mortality, cardiac death, nonfatal myocardial infarction and stroke. A total of 807 patients (mean age 60 ± 10 years, 522 males) received follow-up for 5.1 ± 2.4 years. The rate of all-cause mortality was significantly higher in the large difference group compared to the small difference group (6.6 vs. 2.8 %; adjusted hazard ratio (HR) 2.43; 95 % confidence interval (CI) 1.22–4.83; p = 0.012). There were higher cardiac deaths seen in the large difference group compared to the small difference group (3.9 vs. 1.4 %; adjusted HR 2.84; 95 % CI 1.1–7.31; p = 0.031). Stroke (2.4 vs. 1.2 %, p = 0.125) and TVR (3.7 vs. 1.7 %, p = 0.051) had higher trends in the large difference group compared to the small difference group. The composite of primary endpoints (all-cause mortality, cardiac death, nonfatal MI and stroke) occurred more frequently in the large difference group compared to the small difference group (10.0 vs. 6.4 %; adjusted HR 1.71; 95 % CI 1.04–2.81; p = 0.033). A difference in ward-to-cath lab systolic BP of >20 mmHg may contribute to increased adverse outcomes in the form of all-cause mortality and cardiac deaths in patients undergoing PCI with DES.

Similar content being viewed by others
References
Cheung AT (2006) Exploring an optimum intra/postoperative management strategy for acute hypertension in the cardiac surgery patient. J Card Surg 21(Suppl 1):S8–S14
Aronson S, Boisvert D, Lapp W (2002) Isolated systolic hypertension is associated with adverse outcomes from coronary artery bypass grafting surgery. Anesth Analg 94(5):1079–1084
Aronson S, Stafford-Smith M, Phillips-Bute B, Shaw A, Gaca J, Newman M (2010) Cardiothoracic anesthesiology research endeavors intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology 113(2):305–312
Howell SJ, Sear JW, Foex P (2004) Hypertension, hypertensive heart disease and perioperative cardiac risk. Br J Anaesth 92(4):570–583
Gottesman RF, Hillis AE, Grega MA, Borowicz LM Jr, Selnes OA, Baumgartner WA, McKhann GM (2007) Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Arch Neurol 64(8):1111–1114
Cernaianu AC, Vassilidze TV, Flum DR, Maurer M, Cilley JH Jr, Grosso MA, DelRossi AJ (1995) Predictors of stroke after cardiac surgery. J Card Surg 10(4 Pt 1):334–339
Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A (2001) ELSA investigators relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European lacidipine study on atherosclerosis (ELSA). J Hypertens 19(11):1981–1989
Cay S, Cagirci G, Demir AD, Balbay Y, Erbay AR, Aydogdu S, Maden O (2011) Ambulatory blood pressure variability is associated with restenosis after percutaneous coronary intervention in normotensive patients. Atherosclerosis 219(2):951–957
Jones A, Steeden JA, Pruessner JC, Deanfield JE, Taylor AM, Muthurangu V (2011) Detailed assessment of the hemodynamic response to psychosocial stress using real-time MRI. J Magn Reson Imaging 33(2):448–454
Thygesen K, Alpert JS, White HD (2007) Universal definition of myocardial infarction. Eur Heart J 28(20):2525–2538
Kario K, Schwartz JE, Gerin W, Robayo N, Maceo E, Pickering TG (2002) Psychological and physical stress-induced cardiovascular reactivity and diurnal blood pressure variation in women with different work shifts. Hypertens Res 25(4):543–551
Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G (2002) Blood pressure variability and organ damage in a general population: results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension 39(2 Pt 2):710–714
Veerman DP, de Blok K, van Montfrans A (1996) Relationship of steady state and ambulatory blood pressure variability to left ventricular mass and urinary albumin excretion in essential hypertension. Am J Hypertens 9(5):455–460
Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y (2000) Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 36(5):901–906
Estafanous FG, Tarazi RC (1980) Systemic arterial hypertension associated with cardiac surgery. Am J Cardiol 46(4):685–694
Granger DN (1999) Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation 6(3):167–178
Herskowitz A, Mangano DT (1996) Inflammatory cascade. A final common pathway for perioperative injury? Anesthesiology 85(5):957–960
Hennein HA, Ebba H, Rodriguez JL, Merrick SH, Keith FM, Bronstein MH, Leung JM, Mangano DT, Greenfield LJ, Rankin JS (1994) Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg 108(4):626–635
Kobzar G, Mardla V, Ratsep I, Samel N (2006) Platelet activity before and after coronary artery bypass grafting. Platelets 17(5):289–291
Leslie JB (1993) Incidence and aetiology of perioperative hypertension. Acta Anaesthesiol Scand Suppl 99:5–9
Hebert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I (2001) Transfusion requirements in critical care investigators for the canadian critical care trials group is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med 29(2):227–234
Aessopos A, Deftereos S, Farmakis D, Corovesis C, Tassiopoulos S, Tsironi M, Georgonikou D, Moyssakis J (2004) Cardiovascular adaptation to chronic anemia in the elderly: an echocardiographic study. Clin Invest Med 27(5):265–273
Ishii S, Inomata T, Ikeda Y, Nabeta T, Iwamoto M, Watanabe I, Naruke T, Shinagawa H, Koitabashi T, Nishii M, Takeuchi I, Izumi T (2014) Clinical significance of heart rate during acute decompensated heart failure to predict left ventricular reverse remodeling and prognosis in response to therapies in nonischemic dilated cardiomyopathy. Heart Vessels 29(1):88–96
Sunbul M, Seckin D, Durmus E, Ozqen Z, Bozbay M, Bozbay A, Kivrak T, Oquz M, Sari I, Erqun T, Aqirbasli M (2014) Assessment of arterial stiffness and cardiovascular hemodynamics by oscillometric method in psoriasis patients with normal cardiac functions. Heart Vessels. doi:10.1007/s00380-014-0490-y
Nagai M, Kario K (2013) Visit-to-visit blood pressure variability, silent cerebral injury, and risk of stroke. Am J Hypertens 26:1369–1376
Acknowledgments
This study was supported by 2013 Research Grant from Kangwon National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Her, AY., Ann, S.H., Lee, J.H. et al. Differences in ward-to-cath lab systolic blood pressure predicts long-term adverse outcomes after drug-eluting stent implantation. Heart Vessels 30, 740–745 (2015). https://doi.org/10.1007/s00380-014-0550-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0550-3