Abstract
Climate change-related warming and increased precipitation may alter winter snow cover and thawing events, and therefore, may carry significant consequences for nitrous oxide (N2O) production pathways such as denitrification, and the abundance and expression of denitrifying microorganisms. We used a soil microcosm study to investigate the combined effect of soil thaw temperature, initial water filled pore space (WFPS) prior to soil freezing, and snowmelt infiltration simulated by the addition of water on N2O emission and denitrification rates, soil respiration rate, and the abundance and transcription of denitrifying (nirK, nirS, and nosZ) bacteria during a single freeze-thaw event. Soil respiration rate was primarily controlled by an increase in soil thaw temperature, whereas soil N2O emission and denitrification rates were generally greater in soils with a higher initial WFPS and soil thaw temperature. In contrast, snowmelt infiltration generally had a negligible effect on these rates, which may be related to pre-existing soil conditions that were already conducive to denitrification. Unexpectedly, the nosZ transcript/nosZ gene abundance ratio was lower in soils thawed at 8.0 °C compared to 1.5 °C; however, this may have resulted in a lower N2O reduction, thus explaining the greater levels of N2O emitted from soils thawed at 8.0 °C. Overall, this study demonstrated that increased N2O production during a single freeze-thaw event was primarily linked to antecedent conditions of high initial WFPS, soil thaw temperature, and a synergistic interplay between these two environmental parameters, and provides evidence that an increase in annual temperature and precipitation, along with the timing of precipitation, may further stimulate N2O production pathways.
Similar content being viewed by others
Introduction
In Canada, agriculture accounts for 75% of total anthropogenic annual emissions of the potent greenhouse gas nitrous oxide (N2O) (Environment and Climate Change Canada 2023), of which non-growing season fluxes associated with freeze-thaw cycles represent 29–73% of the annual N2O emissions (Risk et al. 2013; Wagner-Riddle et al. 2017; Hung et al. 2021). Snow cover, which insulates soil from changes in air temperature, can affect winter soil nitrogen (N) cycling and soil gaseous emissions (Brin et al. 2018, 2019; Jia et al. 2021). However, recent climate-related increases in annual temperature and precipitation may alter the amount, timing, and continuity of winter snow cover and thawing events (Huntington et al. 2004; Hayhoe et al. 2007; Brooks et al. 2011; Blankinship and Hart 2012). Subsequently, these changes may lead to later snow onset, increased mid-winter snowmelt events, and early spring snowmelt, and therefore, may carry implications for denitrification and N2O production.
Although increased N2O production from a snowmelt event was shown to be partially due to the physical release of trapped gas build-up under ice layers within the soil (Bremner et al. 1980; Burton and Beauchamp 1994; Teepe et al. 2001), substantial emissions arise from de novo N2O production (Wagner-Riddle et al. 2008; Németh et al. 2014; Risk et al. 2014). Snowmelt infiltration increases the soil water content, which subsequently lowers soil oxygen (O2) concentrations and creates anoxic microsites that are suitable for denitrifier activity and N2O production (Teepe et al. 2000; Koponen and Martikainen 2004; Congreves et al. 2018). Snowmelt infiltration also increases substrate availability for N2O production pathways through increased nutrient transport, cell lysis from excessive influx of water into microbial cells, and cytoplasmic solute secretion from osmotic regulation (Christensen and Christensen 1991; Fierer and Schimel 2003; Congreves et al. 2018).
The effect of snowmelt infiltration on N2O emissions during a freeze-thaw event is, however, complex and may be confounded by several factors (Congreves et al. 2018). Intermittent N2O fluxes during freeze-thaw events are also related to changes in soil temperature (Congreves et al. 2018; Brin et al. 2018, 2019; Badewa et al. 2022). Soil warming increases microbial metabolism and anoxic microsite formation that promote N2O producing pathways such as denitrification (Henry 2008, 2013; Chantigny et al. 2019) and increases soil water content that further stimulates microbial activity and denitrification (Phillips 2008; Risk et al. 2013; Congreves et al. 2018).
Soil moisture is another primary control of N2O production during freeze-thaw events, where high soil moisture leads to greater N2O pulses during spring thaw (Chen et al. 2018, 2021; Brin et al. 2019; Li et al. 2021; Badewa et al. 2022). It has been shown that high spring thaw N2O fluxes were mainly due to pre-winter conditions (i.e., soil moisture at the time of soil freezing) rather than spring soil conditions (Banerjee et al. 2016; Brin et al. 2018). These findings were attributed to a greater disruption of soil aggregates and microbes in moist soils during soil freezing that resulted in an increased release of substrates for denitrification (Brin et al. 2018, 2019; Congreves et al. 2018).
Given the interconnection between snowmelt infiltration, soil warming, and soil moisture, there is reason to believe a concomitant change in these biophysical factors will exacerbate N2O production during a freeze-thaw event. Few studies have investigated the combined effect of soil moisture prior to soil freezing and soil thaw temperature on N2O emissions (Koponen and Martikainen 2004; Chen et al. 2021; Yang et al. 2022); however, there are currently no studies that have investigated the interaction between snowmelt infiltration, soil thaw temperature, and soil moisture prior to soil freezing on N2O production and denitrifier abundance and expression during a freeze-thaw event. A better understanding of the mechanisms by which thawing leads to N2O production will help predict changes that may occur with climate change-altered winter snow cover.
The objective of this study was to investigate the effects of soil moisture prior to soil freezing (initial water-filled pore space [WFPS] of 15% or 30%), soil thaw temperature (1.5–8.0 °C), and snowmelt infiltration (simulated by with or without water addition) on soil inorganic N availability, soil respiration, denitrification rate, N2O emissions and denitrifier gene abundance and transcription. We hypothesized that soil under conditions that are more conducive to denitrification, i.e., with a combination of greater WFPS before soil freezing, higher soil thaw temperature, and snowmelt infiltration, would have greater N2O emissions, denitrification rates, denitrifier abundance and denitrifier gene transcription, compared to soils with lower WFPS, lower soil thaw temperature, and no snowmelt infiltration. We also hypothesized that the timing of soil water additions would influence N2O production during thaw, where ‘pre-winter’ soil water conditions (initial WFPS) would have a priming effect on the denitrifier communities, resulting in a greater impact on N2O production than ‘spring’ soil water conditions (snowmelt infiltration).
Materials and methods
Experimental design
This microcosm experiment consisted of a 2 × 2 × 2 factorial arrangement of treatments in a completely randomized design (n = 4). Factors included two initial WFPS treatments before soil freezing [15% (W15) or 30% (W30)], two soil thaw temperatures [1.5 °C (T1.5) or 8.0 °C (T8.0)], and two snowmelt infiltration treatments simulated by water addition [no snowmelt (SM-) or snowmelt (SM+)]. In total, there were eight treatment combinations: W15T1.5SM-, W15T1.5SM+, W15T8.0SM-, W15T8.0SM+, W30T1.5SM-, W30T1.5SM+, W30T8.0SM-, and W30T8.0SM+.
The initial WFPS treatments of 15% and 30% were selected based on the range of volumetric water content observed in snow-covered plots in a field during winter freezing (late December 2013-January 2014) (Brin et al. 2018). Soil thaw temperature treatments of 1.5 °C and 8.0 °C were selected based on the range of mean daily temperatures observed in snow-covered plots in a field during spring thaw (late February-April 2014) (Brin et al. 2018). To implement the snowmelt infiltration treatments, soils were treated with deionized water (0.08 mL dH2O g-1 dry soil; temperature of 0.5 °C). This snowmelt volume was selected based on the volumetric water content observed in snow-covered plots in a field during spring thaw (late February-April 2014) (Brin et al. 2018). The deionized water was added at once, as this was representative of a rapid snow melt frequently observed in the spring (greater atmospheric temperature under sunny or rainy conditions) or during the coldest months of winter, January and February (sudden increase in atmospheric temperature accompanied by abundant rainfall).
Soil sampling
Soil (0–20 cm depth) was collected from a field under a potato (Solanum tuberosum L.)-barley (Hordeum vulgare L.) rotation at the Fredericton Research and Development Centre, Agriculture and Agri-Food Canada located in Fredericton, New Brunswick, Canada (45°55’23”N, 66°36’25”W). The soil texture was a sandy loam (680 g sand kg-1, 314 g silt kg-1, and 66 g clay kg-1) (pipette method with organic matter removal, Kroetsch and Wang 2007). Soil organic C and total N concentrations were 10.3 g C kg-1 and 1.2 g N kg-1, respectively, as determined by dry combustion (Elementar varioMACRO, Skjemstad and Baldock 2007). Soil pH (1:1 water dilution) was 6.0. The soil was collected in early December 2014, air-dried, passed through a 4.75 mm sieve, and held at 4.0 °C until microcosm preparation in late February 2015.
Microcosm preparation and sampling
A schematic of the microcosm preparation and sampling is presented in Fig. 1. While working in a walk-in refrigerator set at 4.0 °C, soil for each replicate was divided into bins designated for the two initial WFPS treatments. To each bin, ground red clover residues were added at a rate of 1000 mg C kg-1 dry soil, which falls within the range of expected organic C additions in agricultural soils after the plow-down of plant residues (Bolinder et al. 2002; Miller et al. 2012). Soil nitrate (NO3-) solution was added to the soil to obtain a concentration of 15 mg N kg-1 dry soil. The red clover residues had a C/N ratio of 14:1 and represented a forage crop that is frequently incorporated into agricultural soils in autumn in eastern Canada as a source of organic matter. Concentrations of added NO3− and organic C were in the range of concentrations encountered under field conditions following autumn crop residue incorporation (Bolinder et al. 2002). The WFPS of soil was determined using the gravimetric water content (GWC) and the following calculation: 100 x (GWC x bulk density)/total soil porosity, where total soil porosity = 1-(bulk density/2.65 g cm-3 (soil particle density). The soil was at an initial WFPS of 15%. The amount of water required to obtain a WFPS of 30% was determined using the same calculation and deionized water was added to the bins for the W30 treatment. Soils of W15 and W30 treatments were well-homogenized in the bins by gently mixing.
Schematic overview of treatment implementation and experimental set-up of the single freeze-thaw event used in this study. Soils were adjusted to an initial water filled pore space (WFPS) of either 15% or 30% (W15 or W30) and incubated at -1.0 °C for four-weeks. Soils were then incubated for a seven-day period at either 1.5 or 8.0 °C and were amended with or without water to simulate snowmelt infiltration (SM+ or SM−). The measurements performed in each jar set are presented under Step 4.
A 500 mL glass canning jar served as an individual soil microcosm. The soil (276 g equivalent of oven-dry soil) was added to the microcosms and then hand-packed to the target bulk density of 1.03 Mg m-3 which is similar to field conditions and ensures similar oxygen concentration and substrate availability among soil microcosms. Microcosms were covered with Parafilm pierced with holes to reduce water evaporation and allow for gas exchange, then placed in incubators (model MIR-553, Sanyo Scientific, Japan) set at -1.0 °C for four weeks. The soil freeze temperature of -1.0 °C was selected based on the maintained soil temperature observed in snow-covered plots in a field during winter freezing until spring thaw (late December 2013-late February 2014) (Brin et al. 2018). After four weeks of winter freezing, microcosms were then moved to an incubator set at 1.5–8.0 °C to implement the soil thaw temperature treatments (T1.5 or T8.0) and were treated with or without deionized water to implement the snowmelt infiltration treatments (SM+ or SM-). Deionized water was added all at once in the soil microcosms. This is representative of field conditions; a sudden increase in atmospheric temperature accompanied by abundant rainfall resulting in an intense snow melt is frequently observed in the spring but can even occur during the coldest months of winter (January and February) in the Maritimes. The addition of snowmelt to W15 and W30 treatments resulted in a WFPS of 30% and 44%, respectively. The WFPS within treatments was stable throughout the incubation (Fig. 2). Soil temperatures were monitored in soil microcosms that were not used for sampling throughout the incubation using iButton technology (Thermochron, Whitewater, WI, USA) by placing an iButton data logger in the soil, and temperature data can be found in the supplemental information (Figure S1a-d).
Soil and gas samples were collected at day 0 (i.e., the end of the four-week freezing period at -1.0 °C) and immediately before the thaw temperature and snowmelt infiltration treatments were imposed. The day 0 time point was used as baseline data for examining the temporal changes in N2O emissions, denitrification rates, soil inorganic N concentration, soil moisture, and denitrifier gene abundance and transcription from day = 0 to the end of the incubation period. Following this, the thaw temperature and snowmelt infiltration treatments were implemented, and three sets of microcosms were used to quantify all desired parameters. The headspace of the first set was frequently non-destructively sampled throughout the incubation period to quantify soil respiration and N2O emissions every 4.8 h (with 9.6 h overnight) from 0 to 3 days and then every 12 h from 3 to 7 days. The second and third sets were destructively sampled at 1, 3, and 7 days, where the second set was used to quantify soil inorganic N concentration, soil WFPS, and denitrifier gene abundance and transcription and the third set to quantify denitrification rate. The sampling frequency and incubation length were selected based on previous studies that observed that the majority of CO2 and N2O emissions occurred within 72 h following soil thawing/ re-wetting and lasted for 7 days of incubation (Priemé et al. 2001; Koponen and Martikainen 2004; Teepe et al. 2004).
Soil respiration, N2O emissions and denitrification rate
At each sampling time point, the first set of microcosms designated for frequent gas sampling were sealed with a screw-top lid fitted with a rubber septum, and 10% of the headspace volume was replaced with compressed air to provide a sufficient volume of gas for sampling. To quantify soil respiration (i.e., emissions of CO2) and N2O emission rates, headspace gas (20 mL) was collected at 0, 30, and 60 min and transferred to a pre-evacuated 12 mL glass vial (Exetainers; Labco, High Wycombe, U.K.). After gas sampling, the microcosms were re-covered with Parafilm pierced with holes.
Rates of total denitrification (N2O + N2) were quantified using the third set of microcosms which were sealed with a screw-top lid at the time of sampling. 10% of the headspace volume was replaced with acetylene (C2H2) to quantify total denitrification. The presence of C2H2 inhibits N2O reductase activity; consequently, N2O emissions quantified in the presence of C2H2 reflect total denitrification as specified by Groffman et al. (2006). A 60-min C2H2 diffusion period (Miller et al. 2008) occurred before the removal of the initial gas sample, and therefore 20 mL of headspace gas was collected at 60, 120, 180, and 240 min after sealing and transferred to a pre-evacuated 12 mL glass vial (Exetainers; Labco, High Wycombe, U.K.).
Headspace gas samples were analyzed for CO2 and N2O concentrations using a Varian Star 3800 gas chromatograph (Varian, Mississauga, ON), as described by Burton et al. (2008). Soil respiration, N2O emissions, and denitrification rates were calculated as the change in the mass of CO2-C or N2O-N in the microcosm headspace over 60 min (frequent sampling) or 180 min (destructive sampling). Cumulative gas fluxes of N2O and CO2 measurements (jar set 1) and denitrification gas flux (jar set 3) were calculated over the 7-day incubation period using the linear trapezoidal method of integration between sampling time points (Burton et al. 2008). For the cumulative denitrification gas flux, it was assumed that the gas flux measured on the sampling date was representative of the average daily flux.
Soil inorganic N concentration and moisture
Soil sub-samples were collected from the second set of microcosms.. Soil inorganic N was extracted by shaking 25 g moist soil with 50 mL 0.5M K2SO4 for 30 min at room temperature (~ 20 °C). Extracts were filtered using vacuum filtration and then stored at -20 °C until analysis. Concentrations of soil NO2--N + NO3--N (expressed as NO3--N) and ammonium (NH4+-N) were measured using a Technicon AutoAnalyzer II system following Technicon Industrial Methods #100–70 W and #98–70 W, respectively (Technicon Industrial Systems, Terrytown, MA). Ammonium fixation is not a concern in this soil given the low amount of clay (66 g kg-1 dry soil) and that it consists mostly of kaolinite, a 1:1 clay mineral. Soil moisture (GWC) was also determined in soils from the second set of microcosms. Sub-samples of moist soil were collected and dried in an oven set at 105 °C for 3 days.
DNA and RNA extractions
Soil sub-samples (~ 10 g) were taken from the second set of microcosms, flash frozen in liquid N2 then stored at -80 °C. Both DNA and RNA were extracted from 1 g of freeze-dried soil using the method of Griffiths et al. (2000) and were further purified using the Power Clean DNA Clean-up kit (Mo-Bio, Carlsbad, CA) for DNA using a DNase I treatment and the RNeasy mini kit (Qiagen, Toronto, ON, Canada) for RNA. DNA and RNA were quantified using Picogreen and Ribogreen kits (Invitrogen, Burlington, ON, Canada). Reverse transcription of RNA to cDNA was performed using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) and 0.2 mg mL-1 of BSA using the following conditions: 25 °C for 10 min, 42 °C for 72 min, and 85 °C for 5 min.
Quantitative PCR
Copy numbers of nirS, nirK, and nosZ clade I and the transcript abundance of nosZ clade I were measured by qPCR on DNA and cDNA samples as described by Dandie et al. (2011) with concentration of templates ranging from 2 to 14 ng and 0.2 to 0.4 ng for DNA and cDNA, respectively, using a StepOne Plus Real-time PCR system (Applied Biosystems, Streetsvilles, ON, Canada). Absence of DNA contamination in RNA samples were verified by qPCR on non-reverse transcribed RNA for a subset of samples. Standards were linearized plasmids containing cloned nirS, nirK, and nosZ gene sequences as described in Dandie et al. (2011) and curve descriptors were as follows: for nirS copy numbers: slope = -3.7 to -3.9, efficiency (E) = 80.9 to 85.8%, intercept (Y) = 46.5 to 47.6, and R2 = 0.998; for nirK copy numbers: slope = -3.5 to -3.7, efficiency (E) = 83.3 to 90.9%, intercept (Y) = 43.6 to 48.6, and R2 = 0.998 to 1.00; for nosZ clade I copy numbers: slope = -3.4 to -3.7, efficiency (E) = 87.5 to 88.3%, intercept (Y) = 43.6 to 44.6, and R2 = 0.991 to 0.997; and for nosZ clade I transcript numbers: slope = -3.5 to -3.8, efficiency (E) = 83.7 to 91.3%, intercept (Y) = 43.9 to 44.7, and R2 = 0.994 to 0.997.
Statistical analyses
The RStudio base statistic software was used to conduct statistical analyses (v.4.1.3). Data were assessed for normality (i.e., normal and independent distribution) using homoscedasticity diagnostic plots and Shapiro-Wilks tests. For the frequent gaseous emission rates, a repeated measures ANOVA was performed using initial WFPS, thaw temperature, snowmelt, and time (repeated measure; 20 sampling time points) as fixed factors. ANOVAs for destructive denitrification rate, soil inorganic N concentrations, gene abundances, and the ratio of nosZ transcript / nosZ gene copy number were performed using initial WFPS, thaw temperature, snowmelt, and time (three sampling time points) as fixed factors. Treatment, time, and interaction means were tested using post-hoc Tukey Honest Significant Difference (HSD) tests. The treatment means and standard errors presented in figures were calculated from untransformed data. Significance was accepted at P ≤ 0.05.
Results
Soil inorganic N concentrations
Soil NH4+-N concentration did not vary with initial WFPS at day 0 i.e., at the end of the four-week freezing period and before the thaw temperature and snowmelt infiltration treatments were imposed (Fig. 3a). There was a significant initial WFPS x thaw temperature x time interaction on soil NH4+-N concentration (P = 0.021; Table S1). Soil NH4+-N concentration increased over time for all treatments, and the increase over time was greater for the T8.0 thaw temperature than for the T1.5 thaw temperature, whereas the effect of initial WFPS was somewhat inconsistent, with high initial WFPS increasing NH4+-N concentration only on day 7 for the T1.5 thaw temperature and only on day 3 for the T8.0 thaw temperature (Fig. 3a). There was no significant effect of snowmelt on soil NH4+-N concentration (Table S1).
Soil ammonium (mg N kg− 1 dry soil) and nitrate (mg N kg− 1 dry soil) concentration throughout the seven-day incubation of a single-freeze thaw event. Soils were adjusted to an initial water filled pore space (WFPS) of either 15% or 30% (W15 or W30) and incubated at -1.0 °C for four-weeks. Soils were sampled at day 0 i.e. at the end of the freezing period and immediately before the treatments were imposed. Soils were then incubated for a seven-day period at either 1.5 or 8.0 °C and were amended with or without water to simulate snowmelt infiltration (SM+ or SM−). Values represent the mean (n = 4) and the standard error.
Soil NO3−-N concentration at day 0 was lower for the W30 treatments compared with the W15 treatments (Fig. 3b). There was a significant initial WFPS x thaw temperature interaction on soil NO3−-N concentration (P = 0.021; Table S1). The decrease in soil nitrate due to high initial WFPS was greater at T8.0 thaw temperature than at T1.5 thaw temperature (Fig. 3b). There was also a significant initial WFPS x snowmelt interaction on NO3−-N concentration (P = 0.003; Table S1) where there was a greater decrease in NO3−-N concentration for the low initial WFPS than for the high initial WFPS in the presence of snowmelt (Fig. 3b). In addition, there was a significant main effect of time on soil NO3−-N concentration (P < 0.001; Table S1), in which soil nitrate concentrations significantly decreased over time for all treatments (Fig. 3b).
Soil respiration, N2O emissions, and denitrification rate
Non-destructive sampling for soil respiration and N2O emissions
There was a significant initial WFPS x thaw temperature x time interaction on soil respiration (P < 0.001; Table S2). Soil respiration at T8.0 thaw temperature was approximately double that of the T1.5 thaw temperature and was generally similar regardless of initial WFPS (Fig. 4a). Soil respiration for the W30 treatment at the T8.0 thaw temperature was greater than for the W15 treatment for about the first two days, after which soil respiration was generally similar across initial WFPS treatments and relatively consistent over time. Soil respiration at the T1.5 thaw temperature changed little over time (Fig. 4a). There was also a significant main effect of snowmelt, where the addition of snowmelt increased soil respiration rate (P < 0.001; Table S2).
Soil respiration rate (CO2 mg C kg− 1 dry soil day− 1) and nitrous oxide emission rate (N2O µg N kg− 1 dry soil day− 1) throughout the seven-day incubation of a single-freeze thaw event. Soils were adjusted to an initial water filled pore space (WFPS) of either 15% or 30% (W15 or W30) and incubated at -1.0 °C for four-weeks. Soils were then incubated for a seven-day period at either 1.5 or 8.0 °C and were amended with or without water to simulate snowmelt infiltration (SM+ or SM−). Values represent the mean (n = 4) and the standard error.
There was a significant interaction between initial WFPS x thaw temperature on N2O emissions (P < 0.001, Table S2). Soil N2O emission rates were significantly greater in the W30T8.0 treatment compared to the W30T1.5, W15T1.5, and W15T8.0 treatments, and in the W30T1.5 treatment compared to the W15T1.5 and W15T8.0 treatments (Fig. 4b). There was also a significant interaction between initial WFPS x time (P < 0.001; Table S2) where N2O emission rates increased over time from 2 to 7 days in the W30 treatment but not the W15 treatment.
Destructive sampling for denitrification rate
Denitrification rate at the end of the frozen period was 95% greater across the W30 treatments compared to the W15 treatments (Fig. 5). There was a significant interaction between initial WFPS x thaw temperature on denitrification rate (P < 0.001, Table S3). Denitrification rate for the T8.0 treatment increased over the duration of the incubation for the W30 treatment but remained low over the duration of the incubation for the W15 treatment. For the T1.5 treatment, denitrification rate decreased between day 1 and 3 and remained low thereafter for the W30 treatment but remained low for the entire duration of incubation for the W15 treatment (Fig. 5). Snowmelt did not significantly affect denitrification rate (Table S3).
Denitrification rate (N2O µg N kg− 1 dry soil day− 1) using the acetylene inhibition method throughout the seven-day incubation of a single-freeze thaw event. Soils were adjusted to an initial water filled pore space (WFPS) of either 15% or 30% (W15 or W30) and incubated at -1.0 °C for four-weeks. Soils were sampled at day 0 i.e. at the end of the freezing period and immediately before the treatments were imposed. Soils were then incubated for a seven-day period at either 1.5 or 8.0 °C and were amended with or without water to simulate snowmelt infiltration (SM+ or SM−). Values represent the mean (n = 4) and the standard error.
Cumulative gas emissions
Soil thaw temperature, initial WFPS and snowmelt all significantly affected cumulative soil respiration (both P ≤ 0.001; Table S4). When averaged across other treatments, cumulative respiration was almost twice as great for T8.0 treatments compared to T1.5 treatments, 14% greater for SM+ treatments compared to the SM− treatments, and 8% greater for W30 treatments compared to W15 treatments (Table 1).
There was a significant initial WFPS x thaw temperature x snowmelt interaction on cumulative N2O emissions (P < 0.001; Table S4). Cumulative N2O emissions were low for the W15 treatments regardless of thaw temperature and snowmelt treatments, whereas for the W30 treatment, cumulative N2O emissions were over two-fold greater for T8.0 treatments compared to T1.5 treatments, but 17% lower for SM+ treatments compared to SM- treatments (Table 1).
There was also a significant initial WFPS x thaw temperature x snowmelt interaction on cumulative denitrification emissions (P < 0.001; Table S4). Cumulative denitrification emissions were minimal for the W15 treatments regardless of thaw temperature or snowmelt treatments, whereas for the W30 treatments, cumulative denitrification was about 7 times greater for T8.0 treatments compared T1.5 treatments and the presence of snowmelt decreased denitrification only for the W30T8.0 treatments (Table 1).
Denitrifier abundance and transcription
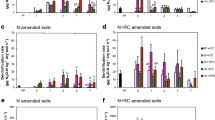
The abundance of nirK, nirS, and nosZ genes and the ratio of nosZ transcript abundance/nosZ gene abundance did not statistically vary with initial WFPS at the day 0. There were significant main effects of soil thaw temperature and time on nirK abundance (both P < 0.001; Table S5). The abundance of nirK genes was significantly greater in the T8.0 treatments compared to the T1.5 treatments and increased over time during the incubation (Fig. 6a). In contrast, while nirS abundance also increased over time, there were no significant effects of initial WFPS, thaw temperature or snowmelt on nirS abundance (P = 0.019; Table S5) (Fig. 6b).
Soil gene abundance (# of copies g− 1 dry soil) of nirK (a), nirS (b), and nosZ clade I (c), and the ratio of nosZ clade I cDNA to nosZ clade I DNA (d) throughout the seven-day incubation of a single-freeze thaw event. Soils were adjusted to an initial water filled pore space (WFPS) of either 15% or 30% (W15 or W30) and incubated at -1.0 °C for four-weeks. Soils were sampled at day 0 i.e. at the end of the freezing period and immediately before the treatments were imposed. Soils were then incubated for a seven-day period at either 1.5 or 8.0 °C and were amended with or without water to simulate snowmelt infiltration (SM+ or SM−). Values represent the mean (n = 4) and the standard error.
The nosZ gene abundance was significantly greater in the W15 treatments compared to the W30 treatments (P < 0.001; Table S6) (Fig. 6c). In addition, there were significant main effects of soil thaw temperature and time on the nosZ transcript abundance/nosZ gene abundance ratio (both P < 0.01; Fig. 6d; Table S6). The nosZ transcript abundance/nosZ gene abundance ratio was significantly greater in the T1.5 treatments compared to the T8.0 treatments and decreased over time.
Discussion
This study investigated the effects of initial WFPS prior to soil freezing, soil thaw temperature, and snowmelt infiltration simulated by water addition on soil respiration, soil N2O emissions, denitrification, and denitrifier abundance and expression during a single soil freeze-thaw event. It was hypothesized that the combination of high initial WFPS before soil freezing, high soil thaw temperature, and snowmelt infiltration during soil thawing would concomitantly create soil conditions conducive to increasing N2O emissions, denitrification, denitrifier abundance and transcription, and that pre-freezing soil water conditions would have a greater influence of N2O production than water added to the soil via snowmelt infiltration during soil thaw.
Effects of initial WFPS, thaw temperature and snowmelt on soil respiration and soil inorganic N concentrations
In this study, respiration rate and cumulative respiration were most affected by thaw temperature and were almost twice as great when thawed at 8.0ºC than when thawed at 1.5ºC. Increased soil water content, either through the addition of water to simulate snowmelt or increased initial WFPS, also resulted in increased cumulative respiration. These findings are similar to previous incubation and field-based studies that observed greater CO2 emissions from soils that had a greater soil water content and temperature at the time of soil thawing or experienced snow accumulation during the winter (Ouyang et al. 2015; Brin et al. 2018; Wertz et al. 2018; Badewa et al. 2022). The increased respiration rate observed in the current study during soil thawing was likely primarily a result of increased heterotrophic metabolism in response to soil warming (Sharma et al. 2006; Feng et al. 2007; Henry 2008, 2013; Chantigny et al. 2019), and increased C substrate availability through a high initial WFPS prior to soil freezing and snowmelt infiltration that further stimulated microbial activity (Christensen and Christensen 1991; Fierer and Schimel 2003; Brooks et al. 2011; Congreves et al. 2018).
Soil NH4-N concentration was primarily affected by soil thaw temperature and was ~ 4-fold greater at the end of the incubation when thawed at 8.0ºC than at 1.5ºC. Similarly, previous studies also found that increased thaw temperature resulted in increased soil NH4-N concentration during a freeze-thaw event (Wertz et al. 2016, 2018; Song et al. 2017; Li et al. 2021). Increased NH4-N concentration in response to a higher thaw temperature was likely associated with an increased net N mineralization following increased microbial metabolism in response to soil warming (Contosta et al. 2011; Grant 2014; Ruan and Roberston, 2017; Song et al. 2017), which is consistent with the observed increase in microbial activity inferred by soil respiration in response to soil warming.
Treatments that resulted in increased soil water content generally resulted in decreased soil NO3-N concentration in soils, likely due to reduced soil oxygen availability creating more anoxic soil conditions, and thereby increasing the rate of denitrification and subsequently NO3− consumption (Firestone et al. 1980; Brin et al. 2018; Congreves et al. 2018). This is supported by the lower NO3-N concentration for the initial WFPS of 30% compared to soil with an initial WFPS of 15%, both at day 0 and throughout the 7 days of incubation. It is also consistent with a reduction in soil NO3-N concentration at 8.0 °C compared to 1.5 °C, as the increased soil thaw temperature would be expected to further promote anoxic microsite formation (Henry 2008, 2013; Chantigny et al. 2019). Interestingly, snowmelt infiltration only decreased NO3-N concentration in soils with an initial WFPS of 15%; this may be due to conditions that were already conducive to denitrification in soils with an initial WFPS of 30% and thus were not significantly enhanced by additional water infiltration.
Effects of initial WFPS, thaw temperature and snowmelt on N2O and denitrification emissions
Soil N2O emission and denitrification rates were generally greater when the soil oxygen supply was more limited at an initial WFPS of 30% and a soil thaw temperature of 8.0 °C, most notably resulting in a ~ 100-fold increase in denitrification rates compared to soils with an initial WFPS of 15% and a soil thaw temperature of 1.5 °C. This was likely related to a combination of interrelated mechanisms that increased substrate availability and anaerobiosis (Fig. 7). This includes the release of substrates through microbial cell lysis, microbial cytoplasmic release, and soil pore connectivity via increased soil moisture (Christensen and Christensen 1991; Fierer and Schimel 2003; Wagner-Riddle, 2017; Congreves et al. 2018); increased microbial metabolism via increased substrate availability and soil warming (Henry 2008, 2013; Chantigny et al. 2019); and increased anoxic microsite formation via increased microbial metabolism and soil moisture (Phillips 2008; Risk et al. 2013; Congreves et al. 2018).
Schematic of the interrelated factors influencing the production of nitrous oxide (N2O), carbon dioxide (CO2), and denitrification rate in soil with an initial soil water-filled pore space (WFPS) of 30% and a soil thaw temperature of 8.0 °C compared to soil with an initial WFPS of 15% and soil thaw temperature of 1.5 °C. Soils with an initial WFPS prior to soil freezing of 15% or 30% were amended with red clover (1000 mg C kg− 1 dry soil) and KNO3− (15 mg N kg− 1 dry soil) and frozen at -1.0 °C for four weeks to simulate winter conditions. The soils were then treated with or without water to simulate the presence (or absence) of snowmelt infiltration and were incubated at 1.5 or8.0 °C for 7 days. Soil N2O, CO2, and denitrification rates were generally greater in soils with an initial WFPS of 30% and a soil thaw temperature of 8.0 °C, resulting in a ~ 100-fold increase in denitrification rates compared to soils with an initial WFPS of 15% and a soil thaw temperature of 1.5 °C. This was likely related to a combination of interrelated mechanisms that increased substrate availability and anaerobiosis. This includes the release of substrates through microbial cell lysis, microbial cytoplasmic release, and soil pore connectivity via increased soil moisture, increased microbial metabolism via increased substrate availability and soil warming, and increased anoxic microsite formation via increased microbial metabolism and soil moisture. The addition of water to simulate snowmelt infiltration did not contribute largely to increasing denitrification, N2O, and CO2 emissions, while increased soil thaw temperature had the greatest role on increased CO2 emissions. However, antecedent conditions of high soil moisture prior to soil freezing was the most important factor in the increased N2O and denitrification rates, indicating that it is not just about soil moisture conditions but also about the timing of water additions to the soil over the non-growing season that influences N2O fluxes.
Interestingly, while the addition of water to simulate snowmelt infiltration would also be expected to reduce oxygen supply and thus increase denitrification and N2O emissions, this was not observed in the current study. Instead, it was primarily the antecedent conditions of high soil moisture prior to soil freezing that gave rise to increased N2O emissions. This supports our original hypothesis that it is not just soil moisture conditions but also the timing of water additions to the soil over the non-growing season that influences N2O fluxes. This is of particular interest when considering the expected increased frequency of freeze-thaw cycles in response to climate warming. For example, if snowmelt infiltration occurred during a thaw event, it may not significantly influence N2O emissions; however, if snowmelt infiltration occurred during a mid-winter thaw event in which the soil re-freezes, then it will further increase soil moisture at the time of soil freezing and carry a significant impact for N2O emissions during spring thaw. Previous investigations have also demonstrated that it is specifically the antecedent conditions of high soil moisture prior to soil freezing that led to increased N2O emissions during a thaw event (Banerjee et al. 2016; Brin et al. 2018), even after 165 freeze-thaw cycles (Chen et al. 2021). A legacy effect of high initial WFPS leading to greater N2O emissions during soil thawing may be related to a greater disruption of soil microbes or soil aggregates upon soil freezing in response to volume expansion that occurs during the phase change from liquid water to ice (~ 9%), subsequently increasing macropore connectivity and nutrient availability for N2O production pathways (Bullock et al. 1988; Christensen and Christensen 1991; Six et al. 2004; Chai et al. 2014; Congreves et al. 2018).
The negligible or insignificant effect of snowmelt infiltration on N2O production may at first be related to pre-freezing soil conditions that may limit snowmelt infiltration. For example, snowmelt infiltration can occur in the open pores of the frozen soil layer; however, if those pores are plugged with ice from high pre-freezing soil water content, then snowmelt infiltration would be restricted or limited during early stages of soil thawing (Gray et al. 1985; Bayard et al. 2005; He et al. 2015; Congreves et al. 2018). However, this can only be the case for the first 24 h (during soil thawing) and not for the remainder of the incubation. Perhaps the negligible effect of snowmelt infiltration on denitrification rate from 1 to 7 days of incubation was due to soil conditions that were already anoxic such that denitrification would not be greatly enhanced by additional water infiltration, or due to snowmelt water containing negligible nitrate or available C, which may act to dilute substrate availability at anoxic microsites.
The C2H2 blockage method may lead to an underestimation of denitrification due to an oxidation reaction between nitric oxide (NO), an intermediate of the denitrification process, and O2 in the presence of C2H2 (Bollmann and Conrad 1997; McKenney et al. 1996). Although there is a possibility of reduced soil O2 concentrations following soil thaw and snowmelt, and thus a reduced possibility of NO oxidation, it is noteworthy that low O2 concentrations have still been shown to increase NO oxidation. (McKenney et al. 1997). Similarly, studies that have added organic C and NO3− to soils reported a significant (McKenney et al. 1996) or minimal (Murray and Knowles 2003) NO oxidation by C2H2 under anoxic conditions. However, lower NO oxidation has been observed in soils incubated at lower temperatures (2 to 10oC) compared to soils incubated at 25oC (Dunfield and Knowles 1997). The lower incubation temperatures used in our study might have reduced the oxidation of NO by C2H2; however, it cannot be ruled out that the denitrification rates were potentially underestimated.
Effects of initial WFPS, thaw temperature and snowmelt on denitrifier abundance and transcription
Similar to denitrification rate, snowmelt infiltration did not affect denitrifier gene abundance or transcription, supporting the theory that water infiltration did not further stimulate denitrification due to the presence of soil conditions already conducive to denitrification. The nirK gene abundance did, however, increase in response to an increase in soil temperature, which is similar to a previous study (Braker et al. 2010), and suggested an increase in microbial metabolism resulting in anoxic soil conditions that were more favorable for denitrification. In contrast, nirS gene abundance did not change in response to the experimental parameters investigated in this study. Our findings are similar to those of Németh et al. (2014) who also observed consistent levels of nirS gene and transcript abundance throughout a spring-thaw event in a field-based study. An increase in nirK but not nirS gene abundance may indicate that nirK-harbouring denitrifiers played a more important role in soil denitrification in our study, which has also been observed in other studies (Yoshida et al. 2009; Kou et al. 2021).
We had hypothesized that greater initial WFPS prior to soil freezing and increased soil thaw temperature would result in a depletion of soil O2 concentration and increased denitrification substrate availability thus favoring the growth of denitrifiers and inducing denitrification. The opposite result was observed: there was a decrease in nosZ gene abundance and transcription in soil with greater initial WFPS prior to soil freezing and increased soil thaw temperature. Our findings contrast those of previous studies, where nosZ gene abundance and transcription increased in response to increased soil moisture or soil warming (Brin et al. 2019; Wu et al. 2020).
In our study, decreased nosZ transcription in warmer soils may have resulted in lower levels of N2O reduction to N2 and thus explain the greater N2O emissions observed in soils thawed at 8.0 °C compared to 1.5 °C. However, decreased nosZ transcription in soils with a greater initial WFPS was inconsistent with the lower proportion of denitrification end-products emitted as N2O in soils with an initial WFPS of 30% compared to 15%. This may reflect high levels of nosZ transcriptional activity in the initial WFPS30 treatments within the first 24 h of the study (i.e., before the first sampling time point), subsequently resulting in a pool of active NOS enzymes and thus low levels of detectable nosZ transcription after 24 h of the incubation. The nosZ gene primer set used in this study might not have captured as much diversity compared to the new primer set developed by Zhang et al. (2021); however, the same subset of the total nosZ denitrifier communities is measured among treatments, thus the results are still informative in understanding how denitrifier abundance is influenced by soil thawing temperature, snow melt and WFPS. It is also worth noting that N2O may have originated from pathways other than denitrification (Gao et al. 2023a, b), potentially explaining the absent relationship between nosZ gene and transcript abundance and increased N2O emissions in our study.
Conclusions
Our study is the first to investigate the synergistic effect of soil moisture at the time of freezing, soil thaw temperature, and snowmelt infiltration on N2O emissions during a freeze-thaw event. In our experimental system, a synergistic effect was observed where a greater response of N2O emissions to increased thaw temperature occurred at a greater initial WFPS. In addition, we observed that snowmelt infiltration only had a modest to negligible effect on N2O emissions and that changes in denitrifier gene or transcript abundance were not associated with N2O emissions or denitrification. As climate change continues to increase annual temperatures and precipitation, it is predicted that winter snow cover and thawing events will become increasingly erratic and discontinuous, leading to increased rain events and reduced snow cover. Our findings unequivocally demonstrate that greater soil moisture at the onset of freezing, caused by amplified rainfall or mid-winter thawing, and higher soil surface thaw temperatures owing to decreased snow cover and elevated atmospheric temperatures, can considerably increase N2O production during a freeze-thaw event. The interactive effects of several factors including a range of soil initial WFPS, freezing temperatures, and snowmelt timing and intensity on denitrifying communities, denitrification rate, and N2O emissions in soil undergoing multiple freeze-thaw events need to be further investigated to better understand how the warmer winters predicted by climate change might influence the soil N dynamics and the production of N2O emissions in the future.
References
Badewa EA, Yeung CC, Rezanezhad F, Whalen JK, Oelbermann M (2022) Spring Freeze–Thaw stimulates Greenhouse Gas Emissions from Agricultural Soil. Front Environ Sci 10:909683. https://doi.org/10.3389/fenvs.2022.909683
Banerjee S, Helgason B, Wang L, Winsley T, Ferrari BC, Siciliano SD (2016) Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol Biochem 95:40–50
Bayard D, Stahli M, Parriaux A, Fluhlerc H (2005) The influence of seasonally frozen soil on the snowmelt runoff at two Alpine sites in southern Switzerland. J Hydrol 309:66–84. https://doi.org/10.1016/j.jhydrol.2004.11.012
Blankinship JC, Hart SC (2012) Consequences of manipulated snow cover on soil gaseous emission and N retention in the growing season: a meta-analysis. Ecosphere 3:1. https://doi.org/10.1890/ES11-00225.1
Bolinder MA, Angers DA, Belanger G, Michaud R, Laverdiere MR (2002) Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Can J Soil Sci 82:731–737
Bollmann A, Conrad R (1997) Acetylene blockage technique leads to underestimation of denitrification rates in oxic soils due to scavenging of intermediate nitric oxide. Soil Biol Biochem 29:1067–1077
Braker G, Schwarz J, Conrad R (2010) Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiol Ecol 73:134–148. https://doi.org/10.1111/j.1574-6941.2010.00884.x
Bremner JM, Robbins SG, Blackmer AM (1980) Seasonal variability in emission of nitrous oxide from soil. Geophys Res Lett 7:641–644. https://doi.org/10.1029/GL007i009p00641
Brin LD, Goyer C, Zebarth BJ, Burton DL, Chantigny MH (2018) Changes in snow cover alter nitrogen cycling and gaseous emissions in agricultural soils. Agric Ecosyst Environ 258:91–103. https://doi.org/10.1016/j.agee.2018.01.033
Brin LD, Goyer C, Zebarth BJ, Burton DL, Chantigny MH (2019) Linking changes in snow cover with microbial nitrogen cycling functional gene abundance and expression in agricultural soil. FEMS Micrbiol Ecol 95:fiz073. https://doi.org/10.1093/femsec/fiz073
Brooks PD, Grogan P, Templer PH, Groffman P, Öquist MG, Schimel J (2011) Carbon and Nitrogen Cycling in Snow-covered environments. Geogr Compass 5:682–699. https://doi.org/10.1111/j.1749-8198.2011.00420.x
Bullock MS, Kemper WD, Nelson SD (1988) Soil cohesion as affected by freezing, water content, time and tillage. Soil Sci Soc Am J 52:770–776
Burton DL, Beauchamp EG (1994) Profile nitrous oxide and carbon dioxide concentrations in a soil subject to freezing. Soil Sci Soc Am J 58:115–122. https://doi.org/10.2136/sssaj1994.03615995005800010016x
Burton DL, Zebarth BJ, Gillam KM (2008) Effect of split application of fertilizer nitrogen on N2O emissions from potatoes. Can J Soil Sci 88:229–239
Chai YJ, Zeng XBESZ, Bai LY, SU SM, Huang T (2014) Effects of freeze–thaw on aggregate stability and the organic carbon and nitrogen enrichment ratios in aggregate fractions. Soil Use Manag 30:507–516. https://doi.org/10.1111/sum.12153
Chantigny MH, Bittman S, Larney FJ, Lapen D, Hunt DE, Goyer C, Angers DA (2019) A multi-region study reveals high overwinter loss of fall-applied reactive nitrogen in cold and frozen soils. Can J Soil Sci 99:126–135. https://doi.org/10.1139/cjss-2018-0151
Chen Z, Yang S, Zhang A, Jing X, Song W, Mi Z, Zhang Q, Wang W, Yang Z (2018) Nitrous oxide emissions following seasonal freeze-thaw events from arable soils in Northeast China. J Integr Agric1 7:231–246. https://doi.org/10.1016/S2095-3119(17)61738-6
Chen Z, Ge S, Zhang Z, Du Y, Yao B, Xie H, Liu P, Zhang Y, Wang W, Zhou H (2021) Soil moisture but not warming dominates Nitrous Oxide emissions during freeze–thaw cycles in a Qinghai–Tibetan Plateau Alpine Meadow with Discontinuous Permafrost. Front Ecol Evol 9:676027. https://doi.org/10.3389/fevo.2021.676027
Christensen S, Christensen BT (1991) Organic matter available for denitrification in different soil fractions: effect of freeze/thaw cycles and straw disposal. J Soil Sci 42:637–647. https://doi.org/10.1111/j.1365-2389.1991.tb00110.x
Congreves KA, Wagner-Riddle C, Si BC, Clough TJ (2018) Nitrous oxide emissions and biogeochemical responses to soil freezing thawing and drying-wetting. Soil Biol Biochem 117:5–15. https://doi.org/10.1016/j.soilbio.2017.10.040
Contosta AR, Frey SD, Cooper AB (2011) Seasonal dynamics of soil respiration and N mineralization in chronically warmed and fertilized soils. Ecosphere 2:1–21
Dandie CE, Wertz S, Leclair CL, Goyer C, Burton DL, Patten CL, Zebarth BJ, Trevors JT (2011) Abundance, diversity and functional gene expression of denitrifier communities in adjacent riparian and agricultural zones. FEMS Microbiol Ecol 77:69–82
Dunfield PF, Knowles R (1997) Biological oxidation of nitric oxide in a humisol. Biol Fert Soils 24:294–300
Environment and Climate Change Canada (2023) National Inventory Report 1990–2021. Greenhouse Gas Sources and Sinks in Canada
Feng X, Nielsen LL, Simpson MJ (2007) Responses of soil organic matter and microorganisms to freeze–thaw cycles. Soil Biol Biochem 39:2027–2037. https://doi.org/10.1016/j.soilbio.2007.03.003
Fierer N, Schimel JP (2003) A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J 67:798–805. https://doi.org/10.2136/sssaj2003.7980
Firestone MK, Firestone RB, Tiedje JM (1980) Nitrous oxide from soil denitrification: factors controlling its. Biol Prod Sci 208:749–751
Gao W, Fan C, Zhang W, Li N, Liu H (2023a) Heterotrophic nitrification of organic nitrogen in soils: process, regulation, and ecological significance. Biol Fert Soils 59:261–274
Gao N, Zhang F, Bo Q, An T, Gao J, Wei X, Yue S, Shen Y, Li S (2023b) Microbial and isotopomer analysis of N2O production pathways in a calcareous film-mulched farmland. Biol Fertil Soils 59:407–422
Grant RF (2014) Nitrogen mineralization drives the response of forest productivity to soil warming: modelling in ecosystems vs. measurements from the Harvard soil heating experiment. Ecol Modell 288:38–46. https://doi.org/10.1016/j.ecolmodel.2014.05.015
Gray DM, Landine PG, Granger RJ (1985) Simulating infiltration into frozen Prairie soils in streamflow models. Can J Earth Sci 22:464–472. https://doi.org/10.1139/e85-045
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid Method for Coextraction of DNA and RNA from Natural Environments for Analysis of Ribosomal DNA and rRNA-Based Microbial Community Composition. Appl Environ Microbiol 66:5488–5491
Groffman PM, Altabet MA, Bohlke JK, Butterbach-Bahl K, David MB, Firestone MK, Giblin AB, Kana TM, Neilsen LP (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appli 16:2091–2122
Hayhoe K, Wake CP, Huntington TG, Luo L, Schwartz MD, Sheffield J, Wood E, Anderson B, Bradbury J, DeGaetano A, Troy TJ, Wolfe D (2007) Past and future changes in climate and hydrological indicators in the US Northeast. Clim Dyn 28:381–407. https://doi.org/10.1007/s00382-006-0187-8
He H, Dyck MF, Si BC, ZhangT, Lv J, Wang J (2015) Soil freezing–thawing characteristics and snowmelt infiltration in Cryalfs of Alberta, Canada. Geoderma Reg 5:198–208. https://doi.org/10.1016/j.geodrs.2015.08.001
Henry HAL (2008) Climate change and soil freezing dynamics: historical trends and projected changes. Clim Change 87:421–434. https://doi.org/10.1007/s10584-007-9322-8
Henry HAL (2013) Soil freezing dynamics in a changing climate: implications for agriculture. In: Ryozo I, Midori Y, Naoyuki M, Thalhammer A, Hincha DK (eds) Plant and microbe adaptations to Cold in a changing World. Springer, New York, NY, pp 89–105. https://doi.org/10.1007/978-1-4614-8253-6.
Hung CY, Ejack L, Whalen JK (2021) Fall-applied manure with cover crop did not increase nitrous oxide emissions during spring freeze-thaw periods. Appl Soil Ecol 158:103786. https://doi.org/10.1016/j.apsoil.2020.103786
Huntington TG, Hodgkins GA, Keim BD, Dudley RW (2004) Changes in the proportion of precipitation occurring as snow in New England (1949–2000). J Clim 17:2626–2636. https://doi.org/10.1175/1520-0442(2004)017%3C2626
Jia Z, Li P, Wu Y, Yang S, Wang C, Wang B, Yang L, Wang X, Li J, Peng Z, Guo L, Liu W, Liu L (2021) Deepened snow cover alters biotic and abiotic controls on nitrogen loss during non-growing season in temperate grasslands. Biol Fertil Soils 57:165–177. https://doi.org/10.1007/s00374-020-01514-4
Koponen HT, Martikainen PJ (2004) Soil water content and freezing temperature affect freeze-thaw related N2O production in organic soil. Nutr Cycl Agroecosyst 69:213–219. https://doi.org/10.1023/B
Kou Y, Liu Y, Li J, Li C, Tu B, Yao M, Li X (2021) Patterns and drivers of Nirk-Type and Nirs-Type Denitrifier Community Assembly. Along Elevation Gradient mSystems 6:e00667–e00621. https://doi.org/10.1128/mSystems.00667-21
Kroetsch D, Wang C (2007) Particle size distribution. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis. CRC, Boca Raton, FL, pp 713–725
Li Y, Shen Y, Wang T (2021) Freezing and thawing cycles affect nitrous oxide emissions in rain-fed lucerne (Medicago sativa) grasslands of different ages. PeerJ 9 Article e12216. https://doi.org/10.7717/peerj.12216
McKenney DJ, Drury CF, Wang SW (1996) Effect of acetylene on nitric oxide production in soil under denitrifying conditions. Soil Sci Soc Am J 60:811–820
McKenney DJ, Drury CF, Wang SW (1997) Reaction of nitric oxide with acetylene and oxygen: implications for denitrification assays. Soil Sci Soc Am J 61:1370–1375
Miller MN, Dandie CE, Zebarth BJ, Burton DL, Goyer C, Trevors JT (2012) Influence of carbon amendments on Soil denitrifier abundance in soil microcosms. Geoderma 170:48–55. https://doi.org/10.1016/j.geoderma.2011.11.022
Murray RE, Knowles R (2003) Production of NO and N2O in the presence and absence of C2H2 by soil slurries and batch cultures of denitrifying bacteria. Soil Biol Biochem 35:1115–1122
Németh DD, Wagner-Riddle C, Dunfield KE (2014) Abundance and gene expression in nitrifier and denitrifier communities associated with a field scale spring thaw N2O flux event. Soil Biol Biochem 73:1–9. https://doi.org/10.1016/j.soilbio.2014.02.007
Ouyang W, Lai X, Li X, Liu H, Lin C, Hao F (2015) Soil respiration and carbon loss relationship with temperature and land use conversion in freeze–thaw agricultural area. Sci Total Environ 533:215–222. https://doi.org/10.1016/j.scitotenv.2015.06.109
Phillips RL (2008) Denitrification in cropping systems at sub-zero soil temperatures. A review. Agron Sustain Dev 28:87–93. https://doi.org/10.1051/agro:2007045
Priemé A, Christensen S (2001) Natural perturbations, drying–wetting and freezing–thawing cycles, and the emission of nitrous oxide, carbon dioxide and methane from farmed organic soils. Soil Biol Biochem 33:2083–2091. https://doi.org/10.1016/S0038-0717(01)00140-7
Risk N, Snider D, Wagner-Riddle C (2013) Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze-thaw cycles. Can J Soil Sci 93:401–414. https://doi.org/10.4141/CJSS2012-071
Risk N, Wagner-Riddle C, Furon A, Warland J, Blodau C (2014) Comparison of simultaneous Soil Profile N2O concentration and surface N2O flux measurements overwinter and at Spring Thaw in an agricultural soil. Soil Sci Soc Am J 78:180–193. https://doi.org/10.2136/sssaj2013.06.0221
Ruan L, Robertson GP (2017) Reduced Snow Cover increases Wintertime Nitrous Oxide (N2O) emissions from an agricultural soil in the Upper U.S. Midwest. Ecosystems 20:917–927. https://doi.org/10.1007/s10021-016-0077-9
Sharma S, Szele Z, Schilling R, Munch JC, Schloter M (2006) Influence of freeze-thaw stress on the structure and function of Microbial communities and denitrifying populations in Soil. Appl Environ Micobiol 72:2148–2154. https://doi.org/10.1128/AEM.72.3.2148-2154.2006
Six J, Bossuyt H, Degryze S, Denefb K (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res 79:7–31. https://doi.org/10.1016/j.still.2004.03.008
Skjemstad JO, Baldock JA (2007) Total and organic carbon. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis. CRC, Boca Raton, FL, pp 225–237
Song Y, Zou Y, Wang G, Yu X (2017) Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: a meta-analysis. Soil Biol Biochem 109:35–49. https://doi.org/10.1016/j.soilbio.2017.01.020
Teepe R, Brumme R, Beese F (2000) Nitrous oxide emissions from frozen soils under agricultural, fallow and forest land. Soil Biol Biochem 32:1807–1810
Teepe R, Brumme R, Beese F (2001) Nitrous oxide emissions from soil during freezing and thawing periods. Soil Biol Biochem 33:1269–1275
Teepe R, Vor A, Beese F, Ludwig B (2004) Emissions of N2O from soils during cycles of freezing and thawing and the effects of soil water, texture and duration of freezing. Eur J Soil Sci 55:357–365
Wagner-Riddle C, Hu QC, Bochove Ev, Schimel J, Jayasundara S (2008) Linking Nitrous Oxide Flux during Spring Thaw to Nitrate denitrification in the Soil Profile. Soil Sci Soc Am J 72:908–916. https://doi.org/10.2136/sssaj2007.0353
Wagner-Riddle C, Congreves KA, Abalos D, Berg AA, Brown SE, Ambadan JT, Gao X, Tenuta M (2017) Globally important nitrous oxide emissions from croplands induced by freeze–thaw cycles. Nat Geosci 10:279–283. https://doi.org/10.1038/NGEO2907
Wertz S, Goyer C, Burton DL, Zebarth BJ, Chantigny MH, Tatti E, Filion M (2016) The amplitude of soil freeze-thaw cycles influences temporal dynamics of N2O emissions and denitrifier transcriptional activity and community composition. Biol Fertil Soils 52:1149–1162. https://doi.org/10.1007/s00374-016-1146-0
Wertz S, Goyer C, Burton DL, Zebarth BJ, Chantigny MH (2018) Processes contributing to nitrite accumulation and concomitant N2O emissions in frozen soils. Soil Biol Biochem 126:31–39. https://doi.org/10.1016/j.soilbio.2018.08.001
Wu X, Chen Z, Kiese R, Fu J, Gschwendter S, Schloter M, Liu C, Butterbach-Bahl K, Wolf B, Dannenmann M (2020) Dinitrogen (N2) pulse emissions during freeze-thaw cycles from montane grassland soil. Biol Fertil Soils 56:959–972. https://doi.org/10.1007/s00374-020-01476-7
Yang Z, She R, Hu L, Yu Y, Yao H (2022) Effects of biochar addition on nitrous oxide emission during soil freeze–thaw cycles. Front Microbiol 12:1033210
Yoshida M, Ishii S, Otsuka S, Senoo K (2009) Temporal shifts in diversity and quantity of nirS and nirK in a rice paddy field soil. Soil Biol Biochem 41:2044–2051. https://doi.org/10.1016/j.soilbio.2009.07.012
Zhang B, Penton CR, Yu Z, Xue C, Chen Q, Chen Z, Yan C, Zhang Q, Zhao M, Quensen JF, Tiedje JM (2021) A new primer set for Clade I nosZ that recovers genes from a broader range of taxa. Biol Fertil Soils 57:523–531
Acknowledgements
Immense thanks to Catherine Clark, Ginette Decker, Karen Terry, Sean Whitney, and Drucie Janes for invaluable help in microcosm preparation, sample collection and processing. This material is based upon work supported by the National Science Foundation Postdoctoral Research Fellowship in Biology to Lindsay Brin [grant number DBI-1308814], and by Agriculture and Agri-Food Canada to Claudia Goyer [Project ID 541/J-000200].
Funding
Open access funding provided by Agriculture & Agri-Food Canada library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sennett, L.B., Brin, L.D., Goyer, C. et al. Effects of soil water content at freezing, thaw temperature, and snowmelt infiltration on N2O emissions and denitrifier gene and transcript abundance during a single freeze-thaw event. Biol Fertil Soils 60, 577–591 (2024). https://doi.org/10.1007/s00374-024-01817-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-024-01817-w