Abstract
Acid-sulphate sugarcane soils in the subtropics are known hot-spots for nitrous oxide (N2O) emissions, yet the reduction of reactive N2O to non-reactive dinitrogen (N2) via specific pathways remains a major uncertainty for nitrogen (N) cycling and loss from these soils. This study investigated the magnitude and the N2O:N2 partitioning of N2O and N2 losses from a subtropical acid-sulphate soil under sugarcane production using the 15N gas flux method, establishing the contribution of hybrid (co- and chemo-denitrification) and heterotrophic denitrification to N2O and N2 losses. Soils were fertilised with potassium nitrate, equivalent to 25 and 50 kg N ha−1, watered close to saturation then incubated over 30 days. An innovative, fully automated incubation system coupled to an isotope-ratio mass-spectrometer enabled real time analysis of 15N2O and 15N2 at sub-diel resolution. Peak losses of N2O and N2 reached 6.5 kg N ha−1 day−1, totalling > 50 kg of N2O+N2-N ha−1. Emissions were dominated by N2, accounting for more than 57% of N2O+N2 losses, demonstrating that the reduction of N2O to N2 proceeded even under highly acidic conditions. Over 40% of N2O, but only 2% of N2 emissions, were produced via hybrid pathways. These findings demonstrate hybrid pathways are generally limited to N2O production, likely driven by high organic matter content and low soil pH, promoting both biotic, and abiotic nitrosation. Regardless of the underlying process, the magnitude of the N2O emissions demonstrates the environmental, but also the potential agronomic significance, of hybrid pathways of N2O formation for N loss from fertilised acid-sulphate soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid sulphate soils (ASS) are commonly found in low-lying coastal areas, characterised by low soil pH (< 4) and the presence of horizons containing sulphidic materials such as pyrite (FeS2). Drainage and disturbance through agricultural practices can expose sulphides to oxygen (O2), leading to the formation of sulphuric acid (H2SO4) and a subsequent decrease in soil pH (Van Breemen 1982). Low soil pH produces a range of detrimental environmental effects such as the mobilisation of toxic metals (Simpson et al. 2010) and poor crop productivity (Melville and White 2020; Michael et al. 2015). Globally, ASS are estimated to cover up to 50 million ha (Fanning et al. 2017; Wang et al. 2022). Despite adverse conditions for agriculture, ASS are often under intensive agricultural production, receiving large amounts of nitrogen (N) fertilisers to sustain crop productivity. This applies for example to ASS within the Ganges delta of India (Sarangi et al. 2022) and the floodplains under sugarcane production on the east-coast of Australia (Linnenluecke et al. 2020). High N availability (Takeda et al. 2021), low soil pH (Liu et al. 2014; Van den Heuvel et al. 2011), and frequent flooding (Hansen et al. 2014) of ASS in low lying coastal areas promote the formation of nitrous oxide (N2O), and extremely high emissions of N2O have been reported from ASS under sugarcane production (Denmead et al. 2010). Nitrous oxide is a long lived and potent greenhouse gas (Prather et al. 2023), and the dominant substance depleting stratospheric ozone (Ravishankara et al. 2009). Over the last decade, N fertiliser inputs to agricultural soils accounted for >50% of the global increase in N2O emissions (Tian et al. 2020). Due to the severe environmental consequences of N2O, research has focussed on processes and mechanisms driving N2O formation in ASS (Macdonald et al. 2011; Mäkelä et al. 2022; Yli-Halla et al. 2020). Few studies have investigated dinitrogen (N2) emissions from ASS (Wang et al. 2022; Xu et al. 2023), and thus the balance between reactive N and environmentally benign N2 remains a major uncertainty for N cycling in ASS.
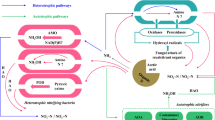
Biotic pathways contributing to production of N2O include nitrifier-denitrification (Stein 2019; Wrage-Mönnig et al. 2018), N2O produced via heterotrophic nitrification (Martikainen 2022), heterotrophic denitrification (henceforth referred to as denitrification) and co-denitrification (Spott and Stange 2011; Tanimoto et al. 1992). Abiotic pathways of N2O production include the chemical decomposition of hydroxylamine (NH2OH) (Heil et al. 2016) and chemodenitrification (Chalk and Smith 1983; Zhu-Barker et al. 2015), the reduction of microbially produced NO2- to N2O via the oxidation of reduced sulphur compounds and/or iron (Fe2+)(Carlson et al. 2013; Chen et al. 2020).
Denitrification but also chemodenitrification are assumed to have NO3− or NO2− as their single source pool of N. By contrast, codenitrification metabolises one N atom of NO2− or nitric oxide (NO) with one atom of a co-substrate via microbially mediated N-nitrosation (Spott et al. 2011). The hybrid formation of N2O and N2 can occur through the formation of an enzyme (E) bound N species (e.g., E-NO and E-NO+). This enzymatic nitrosyl compound can attract nucleophiles such as ammonium (NH4+), NH2OH, hydrazine amino compounds, and ammonia (NH3) to form hybrid N2O or N2 (Clough et al. 2017; Weeg-Aerssens et al. 1988). Codenitrification has been shown to contribute to or even dominate N2O and N2 emissions from pasture soils, particularly under urine patches (Clough et al. 2017; Selbie et al. 2015). The process is ascribed to neutral and alkaline soil pH, while under acidic conditions (pH<5) abiotic nitrosation may also occur. During this process, NO2− and H+ form nitrous acid (HNO2), which can react with NH4+, NH2OH, and amino and other organic N compounds forming N2O and N2 (Spott et al. 2011; Wilson et al. 2021). Anaerobic ammonia oxidation (anammox) is another microbial pathway that co-metabolises oxidised and nucleophile N species. In contrast to biotic and abiotic nitrosation, the final product of this pathway is N2 (Long et al. 2013; Penton et al. 2006).
The 15N gas flux method quantifies N2 emissions following the reduction of a 15N labelled NO3− pool, based on the analysis of N2 and its isotopologues: 28N2 (14N14N), 29N2 (14N15N), and 30N2 (15N15N) (Friedl et al. 2020 and references therein, Mulvaney and Boast 1986). Hybrid formation of N2O and N2 has been discovered through 15N isotope pairing: The provision of 15NO3− or 15NO2− to fungal strains in a pure culture experiment yielded in the formation of 14N15N-N2 (Shoun et al. 1992), revealing the contribution of the 15N labelled mineral N pool, but also the contribution of a non-labelled, organic N pool, forming together hybrid N2O and/or N2. The quantification of N2 via the classic 15N gas flux method as described in Stevens et al. (1993) does not account for hybrid formation of N2 (Spott and Stange 2011), and overall N2 emissions may be underestimated if hybrid pathways contribute to N2 emissions. Hybrid formation of N2 relative to non-hybrid N2 results in a constant numerical ratio (Clough et al. (2001). The incorporation of this ratio in the 15N gas flux method detailed in Selbie et al. (2015), Clough et al. (2017), and Rex et al. (2019) allows to account for the contribution of hybrid pathways of N2O and N2 formation. These calculations are based on the assumption that canonical denitrification contributes to the formation of both 29N2 and 30N2, while hybrid pathways form only 29N2 (Laughlin and Stevens 2002; Long et al. 2013). Hybrid pathways produce one mole of N2O or N2 per mole of NO3−, exceeding the formation potential of canonical denitrification relative to NO3− availability by a factor of two (Butterbach-Bahl et al. 2013a). This highlights the potential environmental significance of hybrid N2O and N2 formation and thus the need to account for these pathways to understand underlying mechanisms of N2O production and the reduction to N2 in fertilised ASS systems.
This study therefore investigated the magnitude and the N2O:N2 partitioning of N2O and N2 emissions from an ASS soil under sugarcane cultivation, while accounting for hybrid pathways that include both abiotic and biotic formation of N2O and N2. The soil for this study was sampled at a location with similar soil properties and close to the field study from Denmead et al. (2010), reporting >30 kg N2O-N ha−1year−1 from an ASS. For this study, we deployed an improved version of an innovative 15N soil incubation system, that enabled real time analysis of N2O and its isotopologues (14N14N16O, 14N15N16O, and 15N15N16O) and N2 at sub diel resolution. Based on the extensive N2O and N2 dataset, we tested the hypothesis that N2O would be the main product of N2O+N2 losses, and that hybrid pathways would form both N2O and N2 emissions from an ASS.
Material and methods
Site description
Soil samples for the experiment were collected on the 15th of January 2020 from an intensively managed sub-tropical sugarcane cropping site in Cudgen, North-Eastern NSW (28° 16′ 01.1″ S 153° 30′ 46.9″ E), Australia. Annual average temperatures range between 14.6 and 24 °C, and rainfall averages 1780 mm yearly (Australian Bureau of Meteorology 2020). The soil is classified as an alluvial hydrosol (Isbell 2016) or Thionic Gleysol (WRB 2015), (commonly referred to as an ASS) with a loamy sandy texture in the 0–100-mm layer, an organic C content of 6.6%, and a soil pH of 4. Selected site characteristics, and soil physical and chemical properties are shown in Table 1. Several studies including Denmead et al. (2010); Macdonald et al. (2011); Wilson et al. (1999), and references therein have investigated N cycling in ASS at close by locations in the Tweed river region in northern NSW.
Sugarcane in the region is grown by planting a part of the mature cane stalks (plant cane), which are harvested annually, and regrown for up to four seasons, referred to as ratoons. Fertiliser N is usually applied as urea when the ratoon crop is approximately 50 cm high at rates between 100 and 160 kg N ha−1 (Denmead et al. 2010; Macdonald et al. 2011).
The selected field was in fallow, having been harvested on the 15th of December 2019, with the top 100 mm tilled. Soil (0–100 mm) was sampled from random positions (n=8) in the field. Overall, approximately 200 kg of fresh soil were collected. The soil was stored for 4 days in a cold room prior to the experiment.
Experimental setup
The experiment included two treatments: 25 and 50 kg NO3−-N ha−1 (henceforth referred to as 25N and 50N treatments) (n=4). Tillage, in combination with cane trash retention at the site, resulted in a high fraction of cane trash in the sampled soil. Large cane trash remnants (> 30 mm) were removed, and the soil was then bulked and loaded into eight soil trays (500 × 500 × 100 mm) at an adjusted bulk density of 0.7 (g cm−3), mimicking conditions in the field. It is noteworthy that due to recent tillage, the BD in the top 10 cm varied considerably, and that the given value of 0.7 is an approximation. Fertiliser N was applied in solution as potassium nitrate (K15NO3, Sigma Aldrich) at the equivalent rate of 25 and 50 kg NO3−-N ha−1 at an enrichment of 60 atom % excess and sprayed onto the soil to ensure even distribution of the 15N label. Additional water was applied to increase the soil water-filled pore space (WFPS) from 70 to 100% on the second day of sampling, simulating an extreme flooding event providing favourable conditions for denitrification. The soil was left to dry via evaporation, and the decrease of WFPS over time was continuously monitored. In total, the soil was incubated for 30 days at a constant temperature of 25 °C.
Automated 15N soil incubation system
Measurements of soil N2O and N2 emissions were taken using an improved laboratory 15N soil incubation setup consisting of a Sercon 20-22 continuous flow isotope ratio mass spectrometer (IRMS) (Sercon, Crewe, UK) coupled to a fully automated soil-gas chamber sampling system via a custom-built trace gas preparation unit (TGP), first introduced in a field trial by Warner et al. (2019). Soil for the system was housed in two banks of four enclosed soil trays (500 × 500 × 10 mm), each paired with a pneumatically actuated static chamber (non-through flow, non-steady state, 500 × 500 × 150 mm). The TPG has two distinct paths, described in detail in Warner et al. (2019). Briefly, N2O is trapped in a cooled zeolite column (0 °C), and released after heating to the IRMS. For N2, the sample is passed through a copper furnace removing excess O2, but also reducing N2O to N2. The sample is then passed on to the IRMS, where N2, together with converted N2O (N2O+N2) are measured as N2. After application of 15N fertiliser, the system enabled real time measurements of the isotopologues of N2O ([14N15N16O + 15N14N16O] and 15N15N16O) and N2 (15N14N, 15N15N), allowing their respective emissions to be calculated. The notation of the isotopologues for N2O here refers to the distribution of isotopes and not to the site preference in the molecule.
Sampling cycle
Fluxes of N2O and N2O+N2 were measured over two daily sampling cycles in which three gas headspace samples were taken over closure periods not exceeding 234 min per chamber. Optimisation of flow rates and sampling time from Warner et al. (2019) allowed two additional daily soil gas measurements per chamber, enabling calculation of two daily fluxes of N2O and N2 per chamber based on the linear increase of 15N2 and 15N2O over three samples. The six chamber gas measurements were nested within intra-sampling reference blocks consisting of two 5 ppm N2O in air standards injected from a certified calibration gas cylinder (Supagas, Beenleigh) along the same path followed by chamber gas samples. Isotopic analysis of a single gas sample injection for N2O and N2 took 17.8 min, allowing a new sample to be run every 18 min. Chamber closure times were optimised by sequentially sampling chambers between two sets of four linked chambers (i.e., 1 and 2 and 5 and 6, 3 and 4 and 7 and 8). At the conclusion of a chamber closure cycle, the chambers were opened, a further reference block measured, and the next two chambers closed at the beginning of the proceeding sampling cycle. Two sampling cycles took 22.2 hours in total, allowing 1.8 hours to retune the IRMS and perform daily maintenance tasks before restarting for the following 22.2 hours.
CO2 monitoring
The system also included an infrared gas analyser (LI-COR 820, LICOR, Lincoln, NE, USA) placed inline of the sampling system to allow measurements of CO2 concentration within each gas sample prior to entering the IRMS. The linear increase of CO2 in the chamber headspace was used as an additional quality check for gas measurements and in identifying potential air leaks in soil-gas chambers prior to the experiment. Due to the low soil pH and the high WFPS, CO2 data was not analysed during the experiment.
Soil mineral N and DOC
Destructive soil samples (9 g, n=4) were taken from additional soil incubation chambers on days 1, 3, 7, 14, 22, and 30 to quantify soil mineral N and dissolved organic C (DOC) concentrations. For mineral N, subsamples were shaken for 1 h with 2M KCl (1:5 w:v) and filtered through Whatman no. 42 filter paper. Extracts were analysed for NO3− and NH4+ with a Thermo Gallery Discreet Analyser (2016). For DOC, subsamples were agitated in 40 ml of DI water for 1 h. The resulting soil/water suspension was centrifuged for 15 min, and the supernatant filtered through 45-μm syringe filters. The filtered sample was then analysed for DOC using a Shimadzu TOC-L analyser (2014). Soil samples were dried at 60 °C, ground to 200 μm and analysed for total soil C and N by IRMS (Sercon 20-22).
Water content and soil O2
Measurements of soil volumetric water content (VWC) and soil O2 were continuously taken from the incubated soil trays over 30 days. Soil VWC (Decagon Instruments, USA) and temperature probes (Onset, USA) were placed at a depth of 40 mm in two of the eight chambers, as well as soil O2 sensors (Apogee Instruments, USA) placed immediately below the soil surface. Soil water and O2 probes were calibrated prior installation.
Calculations and statistical analysis
Fluxes and sources of N2O and N2
The molecular ratios of N2O were calculated using the ion currents (I) at the mass to charge ratio (m/z) 44, 45, and 46 as 45R (45I/44I) and 46R (46I/44I), allowing division of N2O into the fraction of N2O derived from the soil NO3− pool undergoing denitrification d’D of the enrichment aD, and the fraction derived from the pool of natural abundance d’N (Arah 1997; Stevens and Laughlin 2001). Concentrations of N2O were determined using measured (45R,46R) and calculated ratios (47R,48R) of N2O isotopologues relative to those in the 5-ppm reference gas injection (Stevens et al. 1993).
For N2, I at m/z 28, 29, and 30 enabled 29R (29I/28I) and 30R (30I/28I) to be calculated. The differences in 29R and 30R between ambient and enriched atmospheres were expressed as Δ29R and Δ30R. Following the calculations outlined in Clough et al. (2017), fluxes of N2 were calculated as follows:
-
a)
Using Δ29R and Δ30R to calculate 15XN, the soil NO3− pool undergoing denitrification using formula 1 (Mulvaney and Boast 1986)
The fraction of N2 in the chamber headspace derived from denitrification d is then estimated as follows:
-
b)
Using the enrichment of the soil NO3- pool undergoing denitrification derived from the isotopologues of N2O aD as instead of 15XN in formula 2 (Stevens and Laughlin 2001). The 15N atom fraction in the background atmosphere (aa), and in the headspace sample (as), and the molecular fraction of 30N2 in the headspace sample xs allow to calculate aD as:
-
c)
Using Δ29R and Δ30R to calculate the fraction of N2 formed via hybrid pathways (dh) including chemo denitrification and co-denitrification (Clough et al. 2017).
Denitrification can produce increases of 29R and 30R, while hybrid pathways are assumed to contribute mainly to increases of 29R, with a constant ratio of 272 between Δ29R and Δ30R (Clough et al. 2001). Using d, calculated using formula 2, Δ29R attributable to denitrification was calculated solving formula 3 for Δ29R:
The difference between measured and calculated Δ29R was assigned to hybrid pathways. Their contribution to N2 in the headspace was calculated as dh
where Δi is 29R, p1 (0.9963) and q1 (0.0037) are the 14N and 15N atom fractions of the N pool at natural abundance, respectively, and p2 and q2 are the respective fractions of the soil NO3− pool undergoing denitrification. Detailed calculations to derive dh for N2O are given in Rex et al. (2019). Briefly, formula 2 is used where Δi is 45R (45N2O/44N2O), and p1 and q1 are the 14N and 15N atom fractions of the natural abundance pool, respectively, while q2 is the NO3− pool undergoing denitrification of the enrichment aD, and p2 is 1- aD.
Multiplying the respective headspace concentrations with d and dh gave the N2O and N2O+N2 emitted from denitrification (N2Oden and N2Oden+N2den) and hybrid pathways (N2Ohybrid and N2Ohybrid+N2hybrid). Fluxes of N2O and N2O+N2 were calculated based on the slope of linear increase of the respective headspace concentrations over three sampling points, using a coefficient of determination (R2) of 0.8 as a quality check. Fluxes were corrected for air temperature and atmospheric pressure and expressed in g N2O or N2-N ha−1 day−1. Subtracting N2Ohybrid from N2Ohybrid+N2hybrid gave N2 derived from hybrid pathways (N2hybrid), and N2 derived from denitrification (N2den) was calculated as N2Oden+N2den by subtracting the difference of total N2O emitted and N2Ohybrid. Total N2 emitted was calculated as the sum of N2hybrid and N2den. The relative contribution of total N2O to total N2O+N2 losses was calculated as the product ratio N2O/(N2O+N2), irrespective of their pathway of production.
If for a single flux Δ29R and Δ30R values were less than the respective daily detection limit (DL), fluxes of N2 were interpolated. If more than one consecutive flux was below the DL, N2 fluxes were set to 0 until the first reportable flux. Fluxes of N2 below the method detection limit (MDL) were set at ½ daily DL. Fluxes of N2O and N2O+N2 were calculated from the slope of the linear increase in gas concentrations over three measurements. Emissions of N2 were calculated as the difference between N2O and N2O+N2. A coefficient of determination was applied to ensure linearity across the three samples in a closure sequence, and fluxes were discarded if R2 was <0.8. All fluxes were corrected for air pressure and temperature and expressed on an area basis. Details of the calculations can be found in the supplementary material of Warner et al. (2019). Overall, 1440 soil gas samples and 810 reference gas samples were analysed, resulting in 480 flux estimates each of N2O and N2 from eight soil chambers over 30 days. Daily fluxes were calculated as the average of two fluxes. The calculated N2O/(N2O+N2) ratio includes N2O and N2 formed by all pathways.
Statistical analysis
Statistical analysis was conducted with SPSS 22.0 (IBM SPSS Statistics; Version, 2013; Somers, NY) and R (R Core Team and Team 2022). Datasets were tested for normal distribution by Shapiro-Wilks test. Treatment differences for cumulative N2O emissions, N2O hybrid, dN2Ohybrid, N2den, N2hybrid, N2, dN2hybrid, and N2O+N2 and final N2O/(N2O+N2) were investigated using the independent samples t test (P < 0.05). The influence of WFPS, soil NO3−, NH4+, and DOC on dN2Ohybrid, dN2hybrid, and the N2O/(N2O+N2) ratio was quantified using the generalised additive model (GAM) using the R package “mgcViz” (Fasiolo et al. 2020). The GAM model was also used to assess the effect of N application rate and days after fertilisation on the soil NO3-pool undergoing denitrification aD, using the R package “mgcViz”. GAMs are semi-parametric models and have the capability to test and quantify the non-linear relationship between response and explanatory variables. Values in the text and tables represent means ± standard error of the mean.
Results
Performance of the improved incubation setup
The 15N soil incubation system captured two N2O and N2 fluxes day−1 from each of the eight chambers, based on the linear increase of N2O and N2 over three individual measurements of the isotopologues of N2O and N2 via IRMS. The deviation (%) of a single N2 flux from the daily average flux estimate is shown in Fig. 1 for 25N and 50N treatments. Single fluxes of N2 deviated by as much as 97% from the daily average, equal to > 2 kg N2-N ha−1 day−1. This bias was less pronounced in the cumulative N2-N estimates, deviating by up to 12% from cumulative estimates when based on the daily average, amounting to >3.5 kg N2-N ha−1 over the time of the incubation.
The precision of the IRMS for N2 (N2O+N2) at 95% confidence intervals (n=48) for Δ29R was 9.5 × 10−7 and 2.8 × 10−6 for Δ30R. This equated to an MDL for N2 of 18.24 g N ha−1 day−1 with aD assumed at 0.6. The in-batch DL for N2 ranged from 5.5 × 10−7 to 2.1 × 10−6 for Δ29R, and from 2.1 × 10−6 to 3.2 × 10−6 for Δ30R, with 95% of all fluxes exceeding the respective daily DL. The DL for N2O was 1.3 × 10−4 for 45R and 6.3 × 10−5 for 46R. Given the high enrichment of N2O measured against a low background, all measurements exceeded the DL. The DL for the concentration of N2O was 0.11 ppm, which equates to a DL at a 95% confidence interval of 1.5 ppm, resulting in a MDL for N2O of 1.5 g N ha−1 day−1.
Emissions of N2O and N2
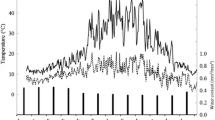
The temporal variability of N2O and N2 fluxes, along with the relative contribution of hybrid pathways (dN2Oh and dN2h), and corresponding measurements of WFPS and soil O2 are shown in Fig. 2. Soil WFPS was adjusted to 100% on day 2 of the experiment and had decreased to 84% by day 30. Soil O2 decreased after day 2 and was below DL by day 15.
Temporal variability of N2O (a) and N2 (c) (kg N ha−1 day−1) and the daily average fraction of N2O (b) and N2 (d) from hybrid pathways from an acid-sulphate sugarcane soil at two different soil NO3− concentrations, plotted against soil WFPS and soil O2 (e) over a 30-day incubation period. The light and dark grey lines show the average N2O and N2 emissions for the 25 and 50 kg NO3−-N ha−1 treatment, respectively, and data points show values for individual replicates. Soil WFPS and O2 are given based on daily averages from continuous monitoring. Fertiliser NO3-N was applied at day 1, and WFPS increased to 100% at day 2
Both the N2O and N2 fluxes increased immediately following the saturation of the soil on day 2. Fluxes of N2O from the 25N treatment peaked at 1147 ± 142 g N2O-N ha−1 day−1 on day 8, while those from the 50N treatment peaked on day 12 at 2249 ± 700 g N2O-N ha−1 day−1. Subsequently, N2O fluxes decreased in both treatments (Fig. 2). Of the total cumulative N2O emissions, 93% and 80% were recorded within the first 15 days, for the 25N and 50N treatment, respectively, with N2O emissions from 50N exceeding those from 25N by a factor >2 (P=0.012) (Table 2). Fluxes of N2 peaked later than those of N2O, equalling 2346 ± 869 g N2-N ha-1 day-1 from the 25N treatment on day 22, and 2867 ± 711 g N2-N ha-1 day-1 from the 50N treatment on day 30 (Fig. 2). Of the total cumulative N2 emissions, 72% and 69% were recorded after day 15, for the 25N and 50N treatment, respectively.
The contribution of hybrid pathways to N2O production over time (dN2O hybrid) ranged from 5 to 89% (Fig. 2). Soil WFPS, NO3− and NH4+ concentration explained 72.1% of the deviance of daily dN2O hybrid values. Values of dN2O hybrid remained stagnant followed by a linear increase with increasing WFPS and responded with an exponential decrease to increasing soil NO3− concentration (Table 3). The contribution of hybrid pathways to N2 production over time (dN2 hybrid) remained below 15% regardless of NO3− addition and showed no response to soil WFPS nor soil mineral N concentrations (Fig. 1, Table 3).
Cumulative emissions of N2O and N2 formed via hybrid pathways (N2O hybrid and N2 hybrid) followed the same trend as overall emissions, with > 80% of total cumulative N2O hybrid, emitted in the first 15 days, and > 60% of cumulative N2 hybrid emissions recorded after day 15 (Table 2).
The N2O/(N2O+N2) ratio exhibited high variability in both treatments and increased to average values ranging from 0.43 to 0.71 after day 1 (Fig. 3). The N2O/(N2O+N2) ratio decreased after day 15 in both treatments, reaching values <0.2 earlier in the 25N treatment than in the 50N treatment.
Effects of WFPS, NO3, and DOC concentrations, analysed with GAMs, explained 92.5% of the deviance in the daily N2O/(N2O+N2) ratios (Table 3): The deviance in the daily N2O/(N2O+N2) ratio had a negative quadratic relationship with change in WFPS, an exponential increase to maximum with increasing soil NO3− concentrations, and a sigmoidal decrease with increasing DOC concentrations.
The 15N enrichment of the soil NO3− pool undergoing denitrification aD (Fig. 4) decreased over time in both treatments and was generally higher in the 50N treatment as compared to the 25N treatment (Table 3).
The 15N fraction of the soil NO3− pool undergoing denitrification ad for the 25 and 50 kg NO3−-N ha−1 treatment over the 30-day incubation period. The light and dark grey lines show the daily average for the 25 and 50 kg NO3−-N ha−1 treatment, respectively, while data points show values for individual replicates
Cumulative N2O emissions from the 50N treatment exceeded those from 25N by a factor of 2.8 (P=0.020), with 8.34 ± 0.26 and 23.11 ± 5.61 kg N2O-N ha-1 emitted over 30 days from the 25N and 50N treatment, respectively. Cumulative N2O hybrid emissions were higher in the 25N treatment (P=0.042) as compared to 50N, accounting for 55% and 40% of cumulative N2O emissions, respectively.
Cumulative N2 emissions did not differ between treatments and accounted for 77 to 57% of total N2O+N2 losses, equivalent to 29.74 ± 3.94 and 30.08 ± 4.70 kg N2-N ha-1, for 25N and 50N, respectively. Cumulative hybrid N2 emissions accounted for only 2% of total N2 emitted, with no differences between treatments. Cumulative losses of N2O+N2 over 30 days totalled in 38.07 ± 3.74 and 53.18 ± 6.36 kg N2O+N2 -N ha-1 and accounted for 152% and 94% of the applied fertiliser N, for the 25N and 50N treatment, respectively.
Soil mineral N and DOC
The addition of KNO3 equivalent to 25 and 50 kg N ha−1 increased soil NO3− concentrations from 33 to 99 and 188 mg NO3−-N in the 25N and 50N treatment, respectively (Fig. 5), and decreased thereafter, with NO3− concentrations remaining < 15 mg NO3−-N after day 15. Soil NH4+ increased over the time of the experiment to > 17 mg NH4+-N in both treatments. The concentration of DOC also increased following the saturation of the soil to >80 mg DOC-C kg−1 soil, decreasing over the time of the experiment to < 30 mg DOC-C kg−1 soil.
Concentrations of soil NO3−, NH4+, and dissolved organic carbon (DOC) (mg kg−1 dry soil) for the 25 and 50 kg NO3−-N ha−1 treatment over the 30-day incubation period. The light and dark grey lines show the daily average for the 25 and 50 kg NO3−-N ha−1 treatment, respectively, while data points are individual replicates
Discussion
Denitrification is a major N loss pathway from ASS
Prolonged periods of flooding are not uncommon for the ASS subject of this study. The experimental conditions are therefore indicative for magnitude and partitioning of N2O and N2 emissions when denitrification rates are expected to peak. Real-time high temporal resolution monitoring of N2O and N2 isotopologues using an innovative 15N soil incubation system demonstrated that more than 50 kg of N2O+N2-N ha−1 were lost over 30 days, indicating denitrification is a major pathway of N loss from these acidic soils. In contrast to conventional soil microcosm incubations, the chamber surface area of the novel incubation system used in this study here is comparable to state of the art greenhouse gas monitoring systems deployed in field studies (Grace et al. 2020). Furthermore, soil incubation in trays prevents subsoil diffusion, and thus avoids underestimation of N2O and N2 emissions (Well et al. 2019). The data presented therefore provide a robust estimate of potential N2O and N2 losses from ASS.
Comparing overall N2O and N2 losses observed with similar studies from pasture soils with high soil organic C content (Friedl et al. 2016), and other sugarcane soils (Kirkby et al. 2023) shows that average daily N2O+N2 losses ranging from 1.3 to 1.7 kg of N2O+N2-N ha−1 day−1 are comparable or even below than those reported in the abovementioned studies. The most notable difference is the extreme high N2O/(N2O+N2) ratio in the study presented here, with N2O accounting for 23 to 43% of overall N2O+N2 losses. The observed N2O/(N2O+N2) ratios are also higher than those observed from a boreal ASS (Xu et al. 2023), which may be explained by higher pH, lack of N addition, or by the use of the acetylene inhibition technique, which can result in an underestimation of N2 (Qin et al. 2012). The N2O/(N2O+N2) ratios presented are similar and higher than those reported after the addition of urea-N at rates of 1000 kg N ha−1 (Clough et al. 2017) to pasture soils with a neutral soil pH. Even though different experimental setups hinder an accurate comparison between incubation studies, differences in soil pH between the abovementioned studies may explain the relatively high N2O/(N2O+N2) ratio from the ASS in the study presented here. The increase of the N2O/(N2O+N2) ratio with decreasing soil pH is widely recognised (Bakken et al. 2012; Liu et al. 2010; Stevens et al. 1998; Zhang et al. 2023), and has been attributed to post transcriptional interference with the expression of nosZ (Frostegård et al. 2022; Liu et al. 2014), the gene encoding the N2O reductase. The continuous N2O+N2 emissions over 30 days, accounting from 100 to 150% of applied fertiliser N, together with the high N2O/(N2O+N2) ratio observed in the study presented here, indicate agronomically important N losses from ASS with severe environmental consequences.
The application of NO3−-N in the study presented here was chosen to enable N2O source partitioning. However, this form of N fertiliser differs from the common application of urea in the region. This is noteworthy, as urea N has to undergo hydrolysis and subsequent nitrification to supply NO3− available for denitrification. Nitrification was assumed to be limited at low soil pH (De Boer and Kowalchuk 2001), yet more recent studies suggest that nitrification proceeds even at a soil pH of 3 (Li et al. 2018). Soil NO3− concentrations of > 30 mg NO3−N kg−1 soil prior to N fertiliser addition observed in our study demonstrate that NO3− is readily available for uptake and loss in this ASS. Nevertheless, the form of N added to the soil may impact on N turnover and thus specific pathways of N2O and N2 production, highlighting the need to expand the findings of the study presented here following the application of urea.
Sources of N2O and N2
To accurately quantify the formation of hybrid N2O and N2, it is necessary to assume that the soil NO3− pool undergoing denitrification is uniformly labelled to avoid artefacts regarding isotope pairing. In the study presented here, the 15N labelled fertiliser was sprayed onto and mixed in with the soil. Together with the high soil WFPS, this setup is likely to ensure a relatively even distribution of the 15N fertiliser (Zaman et al. 2021), enabling accurate estimates of hybrid N2O and N2.
Hybrid formation of N2O accounted for more than 40% of cumulative N2O emissions, yet the reduction to N2 via this pathway was responsible for only 2% of cumulative N2 emissions. The resulting ratio of hybrid N2O:N2 denotes a predominantly truncated pathway driving N2O emissions from this ASS under sugarcane production. This pathway may be explained by denitrifiers lacking the genes encoding the N2O reductase (Philippot et al. 2011), and/or abiotic hybrid processes with N2O as their final product (Spott et al. 2011). It is noteworthy that overall, the reduction of N2O to N2 did proceed throughout the experiment, but was mostly limited to the reduction of non-hybrid N2O. The experimental setup of this study does not allow apportioning between biotic and abiotic pathways that contribute to N2O and N2 emissions. Nevertheless, our findings suggest hybrid formation of N2O as an underlying mechanism for the extremely high N2O emissions observed from ASS in the field (Denmead et al. 2010).
The GAM model showed that the fraction of N2O derived from hybrid pathways, dN2Ohybrid, increased with decreasing NO3− concentration, indicating microbial reduction of NO3− to NO2− via denitrification, supplying NO2− for hybrid N2O formation. These results are in line with the common notion that higher soil NO3− concentrations should provide more NO2− via denitrification, increasing the production of HNO2 under acidic conditions, and therefore the potential for N2O formation via hybrid pathways (Spott et al. 2011; Van Cleemput and Samater 1995). The inverse relationship was shown for NH4+, which may reflect the importance of the co-substrate concentration for nitrosation (Spott et al. 2011; Wilson et al. 2021). The GAM models explained a significant portion of variance for the fraction of N2O derived from hybrid pathways (dN2Ohybrid). However, the explanatory variables for substrate availability are limited in the study presented here, and indicate, in the case of NO3− for example, NO2− substrate availability for hybrid pathways of N2O formation only indirectly. Future research should therefore include the quantification of all relevant intermediates (Lim et al. 2018) to fully capture the kinetics of hybrid N2O formation in ASS. Incorporating such additional data can enhance the GAM’s ability to capture variability, thereby increasing confidence in the model’s output and its applicability to a wider range of conditions.
Both biotic (Butterbach-Bahl et al. 2013b; Yoon et al. 2019) and abiotic (Chalk and Smith 1983, 2020; Zhu-Barker et al. 2015) pathways have been reported to form N2O as their final product. Fungal denitrifiers often lack the N2O reductase (Higgins et al. 2018; Maeda et al. 2015), are less susceptible to acidic conditions than bacteria (Chen et al. 2015; Rousk et al. 2010) and are capable of hybrid N2O formation (Aldossari and Ishii 2021; Laughlin and Stevens 2002; Rex et al. 2019), in particular if the soil matrix is close to or at 100% WFPS (Clough et al. 2017). Abiotic nitrosation via NO+ is likely to occur at a soil pH of 4.0 (Spott et al. 2011), and may therefore have contributed to N2O formation in the study presented here. However, the data obtained do not allow for delineation between abiotic and biotic pathways. The large contribution of hybrid N2O formation observed highlights the need for future research on ASS to tackle the methodological challenges involved with N2O source partitioning between biotic and abiotic pathways (Buessecker et al. 2019; Phillips et al. 2016).
Soil O2 drives N2O:N2 partitioning
The temporal variability of N2O and N2 emissions as well as the N2O/(N2O+N2) ratio responded to increasing O2 limitation in the soil matrix (Fig. 1), with the respective response differing between treatments. Even though soil WFPS is a commonly used proxy for determining decreasing O2 availability, O2 levels were below DL after day 15 of the experiment, despite WFPS being < 100%. These results indicate that microbial O2 consumption lowered the soil redox potential (Azam et al. 2002; Rohe et al. 2021), creating conditions favourable for denitrification. Soil O2 depletion in response to wetting events has been reported from high organic C soils such as pasture soils (Clough et al. 2020; Friedl et al. 2022). This is consistent with high concentrations of DOC (Fig. 5) as a readily available oxidisable C source for microbial availability, amplifying the effect of saturation/flooding on the redox potential of this soil.
Most of the N2O emissions (80% of the total cumulative) were emitted in the first half of the experiment. During this time, N2O emissions dominated (> 50%) cumulative N2O+N2 losses. The positive response of cumulative N2O to NO3− addition reiterates the dual effect of increased NO3− availability: Increased NO3− availability provides more N substrate for denitrifiers, but can also induce preferential NO3− reduction over the reduction of N2O to N2 (Senbayram et al. 2012), with both effects leading to an increase in net N2O emissions. Importantly, the high WFPS during the first 15 days of the experiment did not overwrite the effect of NO3− on N2O emissions as previously reported (Senbayram et al. 2022). Even though abiotic N2O formation cannot be ruled out, the sensitivity of the N2O reductase to the low soil pH (4.0) of the ASS (Kim et al. 2017; Liu et al. 2014; Yoon et al. 2019; Zhang et al. 2023) may also have contributed to the high N2O/(N2O+N2) ratio observed in the first half of the experiment. Our findings indicate that the low soil pH in ASS drives the N2O/(N2O+N2) ratio towards N2O until the depletion of O2 and the soil NO3− pool, even under conditions of complete saturation in the soil matrix.
Increasing the temporal resolution of N2O and N2 measurements
Optimisation of the sampling cycle compared to the field deployed system described in Warner et al. (2019) increased the number of reported fluxes to two fluxes per day from each chamber. The deviation of a single N2 flux from the daily average flux estimate of > 2 kg N2-N ha−1 day−1 (Fig. 1) shows the importance of multiple flux measurements per day to accurately represent the temporal variability of N2 losses. The comparatively low deviation of cumulative estimates of N2 based on a single measurement vs. the daily average likely reflects the rather small changes in soil WFPS during the experiment. This deviation is likely to be larger under conditions of more pronounced wetting and drying cycles, where short-lasting peak flux events account for a large proportion of cumulative N2O and N2 losses (Scheer et al. 2008).
Field and laboratory studies (Čuhel et al. 2010; Higgins et al. 2013; Weier et al. 1998) using manual sampling techniques typically take only two gas samples (T0 and T1) during chamber closure, which precludes the possibility to test for the assumption of linear N2O or N2 gas accumulation within the gas chamber headspace (Friedl et al. 2020). Even if multiple headspace gas samples are taken, N2 fluxes are usually calculated based on two samples (T0 and Ti) (Buchen et al. 2016). The system used in the study presented here, however, offers the advantage of three samples taken during chamber closure, enabling robust quality control to identify linearity and potential analytical artifacts. The data obtained confirms the importance of sampling frequency demonstrated for N2O by Barton et al. (2015) for soil gas exchange of N2O and N2 measured via the 15N gas flux method. The performance of the novel 15N soil incubation system highlights its potential to capture rapid changes of N2O and N2 fluxes together with their respective drivers under both controlled and field conditions.
Conclusions
The first study to account for hybrid N2O and N2 production in ASS demonstrates that under flooded conditions, hybrid pathways contribute substantially to N2O, but not to N2 emissions.
Real-time high temporal resolution monitoring of the isotopologues of N2O and N2 using an innovative 15N soil incubation system showed that N2O dominates denitrification losses in the first 15 days after flooding and N fertilisation in an ASS. Our findings suggest that the low soil pH shifts the N2O/(N2O+N2) ratio towards N2O even under completely flooded conditions. Only as O2 and NO3− levels are depleted after longer periods of flooding does N2 become the main product of denitrification, demonstrated by cumulative N2 emissions, accounting for >57% of overall N2O+N2 losses. The temporal trend of the N2O/(N2O+N2) ratio indicates that unless flooding events persist over extensive periods of time, N2O is likely to be the main product of denitrification from these ASS under sugarcane production. The contribution of hybrid pathways to N2O and N2 formation indicates a truncated pathway of N2O formation, delivering a possible explanation for the high N2O emissions previously reported from this ASS in the field. The inclusion of molecular techniques such as amplicon sequencing and isotope probing in future research may help to disentangle the contribution of specific biotic and abiotic processes that contribute to both hybrid and non-hybrid formation of N2O and N2 emitted from ASS. Regardless of the underlying nature, the magnitude of N2O emissions demonstrates the environmental, but also the potential agronomic significance of hybrid pathways of N2O formation to N loss from fertilised acid-sulphate soils.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aldossari N, Ishii S (2021) Fungal denitrification revisited – Recent advancements and future opportunities. Soil Biol Biochem 157:108250. https://doi.org/10.1016/j.soilbio.2021.108250
Arah JRM (1997) Apportioning nitrous oxide fluxes between nitrification and denitrification using gas-phase mass spectrometry. Soil Biol Biochem 29:1295–1299. https://doi.org/10.1016/S0038-0717(97)00027-8
Australian Bureau of Meteorology (2020) Climate statistics for Australian locations. climate statistics. Available at https://www.bom.gov.au/climate/averages/tables/cw_040717.shtml. Accessed 12 Dec 2023
Azam F, Müller C, Weiske A, Benckiser G, Ottow J (2002) Nitrification and denitrification as sources of atmospheric nitrous oxide – role of oxidizable carbon and applied nitrogen. Biol Fertil Soils 35:54–61. https://doi.org/10.1007/s00374-001-0441-5
Bakken LR, Bergaust L, Liu B, Frostegård Å (2012) Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Philos Trans R Soc Lond B Biol Sci 367:1226–1234. https://doi.org/10.1098/rstb.2011.0321
Barton L, Wolf B, Rowlings D, Scheer C, Kiese R, Grace P, Stefanova K, Butterbach-Bahl K (2015) Sampling frequency affects estimates of annual nitrous oxide fluxes. Sci Rep 5:15912
Buchen C, Lewicka-Szczebak D, Fuß R, Helfrich M, Flessa H, Well R (2016) Fluxes of N2 and N2O and contributing processes in summer after grassland renewal and grassland conversion to maize cropping on a Plaggic Anthrosol and a Histic Gleysol. Soil Biol Biochem 101:6–19. https://doi.org/10.1016/j.soilbio.2016.06.028
Buessecker S, Tylor K, Nye J, Holbert KE, Urquiza Muñoz JD, Glass JB, Hartnett HE, Cadillo-Quiroz H (2019) Effects of sterilization techniques on chemodenitrification and N2O production in tropical peat soil microcosms. Biogeosciences 16:4601–4612. https://doi.org/10.5194/bg-16-4601-2019
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013a) Appendix: nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 368:20130122
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013b) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 368:20130122. https://doi.org/10.1098/rstb.2013.0122
Carlson HK, Clark IC, Blazewicz SJ, Iavarone AT, Coates JD (2013) Fe(II) Oxidation is an innate capability of nitrate-reducing bacteria that involves abiotic and biotic reactions. J Bacteriol 195:3260–3268. https://doi.org/10.1128/jb.00058-13
Chalk P, Smith C (1983) Chemodenitrification. In: Freney JR, Simpson JR (eds) Gaseous loss of nitrogen from plant-soil systems, vol 9. Springer, Dordrecht, pp 65–89. https://doi.org/10.1007/978-94-017-1662-8_3
Chalk PM, Smith CJ (2020) The role of agroecosystems in chemical pathways of N2O production. Agric Ecosyst Environ 290:106783. https://doi.org/10.1016/j.agee.2019.106783
Chen D, Yuan X, Zhao W, Luo X, Li F, Liu T (2020) Chemodenitrification by Fe(II) and nitrite: pH effect, mineralization and kinetic modeling. Chem Geol 541:119586. https://doi.org/10.1016/j.chemgeo.2020.119586
Chen H, Mothapo NV, Shi W (2015) Soil moisture and pH control relative contributions of fungi and bacteria to N2O production. Microb Ecol 69:180–191. https://doi.org/10.1007/s00248-014-0488-0
Clough TJ, Cardenas LM, Friedl J, Wolf B (2020) Nitrous oxide emissions from ruminant urine: science and mitigation for intensively managed perennial pastures. Curr Opin Environ Sustain 47:21–27. https://doi.org/10.1016/j.cosust.2020.07.001
Clough TJ, Lanigan GJ, de Klein CAM, Samad MS, Morales SE, Rex D, Bakken LR, Johns C, Condron LM, Grant J, Richards KG (2017) Influence of soil moisture on codenitrification fluxes from a urea-affected pasture soil. Sci Rep 7:2185. https://doi.org/10.1038/s41598-017-02278-y
Clough TJ, Stevens RJ, Laughlin RJ, Sherlock RR, Cameron KC (2001) Transformations of inorganic-N in soil leachate under differing storage conditions. Soil Biol Biochem 33:1473–1480. https://doi.org/10.1016/S0038-0717(01)00056-6
Čuhel J, Šimek M, Laughlin RJ, Bru D, Chèneby D, Watson CJ, Philippot L (2010) Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl Environ Microbiol 76:1870–1878. https://doi.org/10.1128/aem.02484-09
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866. https://doi.org/10.1016/S0038-0717(00)00247-9
Denmead OT, Macdonald BCT, Bryant G, Naylor T, Wilson S, Griffith DWT, Wang WJ, Salter B, White I, Moody PW (2010) Emissions of methane and nitrous oxide from Australian sugarcane soils. Agric For Meteorol 150:748–756. https://doi.org/10.1016/j.agrformet.2009.06.018
Fanning DS, Rabenhorst MC, Fitzpatrick RW (2017) Historical developments in the understanding of acid sulfate soils. Geoderma 308:191–206. https://doi.org/10.1016/j.geoderma.2017.07.006
Fasiolo M, Nedellec R, Goude Y, Capezza C, Wood SN, Fasiolo MM (2020) Package ‘mgcviz’. In: Visualisations for gener-alised additive models
Friedl J, Cardenas LM, Clough TJ, Dannenmann M, Hu C, Scheer C (2020) Measuring denitrification and the N2O:(N2O+N2) emission ratio from terrestrial soils. Curr Opin Environ Sustain 47:61–71. https://doi.org/10.1016/j.cosust.2020.08.006
Friedl J, Deltedesco E, Keiblinger KM, Gorfer M, De Rosa D, Scheer C, Grace PR, Rowlings DW (2022) Amplitude and frequency of wetting and drying cycles drive N2 and N2O emissions from a subtropical pasture. Biol Fertil Soils 58:593–605. https://doi.org/10.1007/s00374-022-01646-9
Friedl J, Scheer C, Rowlings DW, McIntosh HV, Strazzabosco A, Warner DI, Grace PR (2016) Denitrification losses from an intensively managed sub-tropical pasture – Impact of soil moisture on the partitioning of N2 and N2O emissions. Soil Biol Biochem 92:58–66. https://doi.org/10.1016/j.soilbio.2015.09.016
Frostegård Å, Vick SHW, Lim NYN, Bakken LR, Shapleigh JP (2022) Linking meta-omics to the kinetics of denitrification intermediates reveals pH-dependent causes of N2O emissions and nitrite accumulation in soil. ISME J 16:26–37. https://doi.org/10.1038/s41396-021-01045-2
Grace PR, van der Weerden TJ, Rowlings DW, Scheer C, Brunk C, Kiese R, Butterbach-Bahl K, Rees RM, Robertson GP, Skiba UM (2020) Global research alliance N2O chamber methodology guidelines: Considerations for automated flux measurement. J Environ Qual 49:1126–1140. https://doi.org/10.1002/jeq2.20124
Hansen M, Clough TJ, Elberling B (2014) Flooding-induced N2O emission bursts controlled by pH and nitrate in agricultural soils. Soil Biol Biochem 69:17–24
Heil J, Vereecken H, Brüggemann N (2016) A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur J Soil Sci 67:23–39
Higgins S, Laughlin R, Watson C (2013) Antecedent effect of lime on nitrous oxide and dinitrogen emissions from grassland soils. Nutr Cycling Agroecosyst 95:219–229. https://doi.org/10.1007/s10705-013-9559-8
Higgins SA, Schadt CW, Matheny PB, Löffler FE (2018) Phylogenomics reveal the dynamic evolution of fungal nitric oxide reductases and their relationship to secondary metabolism. Genome Biol Evol 10:2474–2489. https://doi.org/10.1093/gbe/evy187
Isbell R (2016) The Australian soil classification. CSIRO publishing, Collingwood, Victoria
Kim H, Park D, Yoon S (2017) pH control enables simultaneous enhancement of nitrogen retention and N(2)O reduction in Shewanella loihica strain PV-4. Front Microbiol 8:1820. https://doi.org/10.3389/fmicb.2017.01820
Kirkby R, Friedl J, Takeda N, De Rosa D, Rowlings DW, Grace PR (2023) Nonlinear response of N2O and N2 emissions to increasing soil nitrate availability in a tropical sugarcane soil. J Soils Sediments. https://doi.org/10.1007/s11368-023-03482-2
Laughlin RJ, Stevens RJ (2002) Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Sci Soc Am J 66:1540–1548
Li Y, Chapman SJ, Nicol GW, Yao H (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301. https://doi.org/10.1016/j.soilbio.2017.10.023
Lim NYN, Frostegård Å, Bakken LR (2018) Nitrite kinetics during anoxia: the role of abiotic reactions versus microbial reduction. Soil Biol Biochem 119:203–209. https://doi.org/10.1016/j.soilbio.2018.01.006
Linnenluecke MK, Zhou C, Smith T, Thompson N, Nucifora N (2020) The impact of climate change on the Australian sugarcane industry. J Clean Prod 246:118974. https://doi.org/10.1016/j.jclepro.2019.118974
Liu B, Frostegard A, Bakken LR (2014) Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. mBio 5:e01383–e01314. https://doi.org/10.1128/mBio.01383-14
Liu B, Mørkved PT, Frostegård Å, Bakken LR (2010) Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol Ecol 72:407–417
Long A, Heitman J, Tobias C, Philips R, Song B (2013) Co-occurring anammox, denitrification, and codenitrification in agricultural soils. Appl Environ Microbiol 79:168–176
Macdonald BCT, Denmead OT, White I, Byrant G (2011) Gaseous nitrogen losses from coastal acid sulfate soils: a short-term study. Pedosphere 21:197–206. https://doi.org/10.1016/S1002-0160(11)60118-5
Maeda K, Spor A, Edel-Hermann V, Heraud C, Breuil M-C, Bizouard F, Toyoda S, Yoshida N, Steinberg C, Philippot L (2015) N2O production, a widespread trait in fungi. Sci Rep 5:9697. https://doi.org/10.1038/srep09697 http://www.nature.com/articles/srep09697#supplementary-information
Mäkelä M, Kabir KMJ, Kanerva S, Yli-Halla M, Simojoki A (2022) Factors limiting microbial N2O and CO2 production in a cultivated peatland overlying an acid sulphate subsoil derived from black schist. Geoderma 405:115444. https://doi.org/10.1016/j.geoderma.2021.115444
Martikainen PJ (2022) Heterotrophic nitrification – an eternal mystery in the nitrogen cycle. Soil Biol Biochem 168:108611. https://doi.org/10.1016/j.soilbio.2022.108611
Melville MD, White I (2020) Acid sulfate soils: Management. In: Fath BD, Jorgensen SE (eds) Managing soils and terrestrial systems, 2nd edn. CRC Press, Boca Raton, pp 363–371. https://doi.org/10.1201/9780429346255
Michael PS, Fitzpatrick R, Reid R (2015) The role of organic matter in ameliorating acid sulfate soils with sulfuric horizons. Geoderma 255-256:42–49. https://doi.org/10.1016/j.geoderma.2015.04.023
Mulvaney RL, Boast CW (1986) Equations for determination of nitrogen-15 labeled dinitrogen and nitrous oxide by mass spectrometry1. Soil Sci Soc Am J 50:360–363. https://doi.org/10.2136/sssaj1986.03615995005000020021x
Penton CR, Devol AH, Tiedje JM (2006) Molecular evidence for the broad distribution of anaerobic ammonium-oxidizing bacteria in freshwater and marine sediments. Appl Environ Microbiol 72:6829–6832. https://doi.org/10.1128/AEM.01254-06
Philippot L, Andert J, Jones CM, Bru D, Hallin S (2011) Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob Change Biol 17:1497–1504. https://doi.org/10.1111/j.1365-2486.2010.02334.x
Phillips RL, Song B, McMillan AMS, Grelet G, Weir BS, Palmada T, Tobias C (2016) Chemical formation of hybrid di-nitrogen calls fungal codenitrification into question. Sci Rep 6:39077. https://doi.org/10.1038/srep39077
Prather MJ, Froidevaux L, Livesey NJ (2023) Observed changes in stratospheric circulation: decreasing lifetime of N2O, 2005–2021. Atmos Chem Phys 23:843–849. https://doi.org/10.5194/acp-23-843-2023
Qin S, Hu C, Oenema O (2012) Quantifying the underestimation of soil denitrification potential as determined by the acetylene inhibition method. Soil Biol Biochem 47:14–17. https://doi.org/10.1016/j.soilbio.2011.12.019
R Core Team A, Team RC (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, p 2012
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. https://doi.org/10.1126/science.1176985
Rex D, Clough TJ, Richards KG, Condron LM, de Klein CAM, Morales SE, Lanigan GJ (2019) Impact of nitrogen compounds on fungal and bacterial contributions to codenitrification in a pasture soil. Sci Rep 9:13371. https://doi.org/10.1038/s41598-019-49989-y
Rohe L, Apelt B, Vogel HJ, Well R, Wu GM, Schluter S (2021) Denitrification in soil as a function of oxygen availability at the microscale. Biogeosciences 18:1185–1201. https://doi.org/10.5194/bg-18-1185-2021
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. https://doi.org/10.1038/ismej.2010.58
Sarangi SK, Mainuddin M, Maji B (2022) Problems, management, and prospects of acid sulphate soils in the ganges delta. Soil Systems 6:95
Scheer C, Wassmann R, Kienzler K, Ibragimov N, Eschanov R (2008) Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: influence of nitrogen applications and irrigation practices. Soil Biol Biochem 40:290–301. https://doi.org/10.1016/j.soilbio.2007.08.007
Selbie DR, Lanigan GJ, Laughlin RJ, Di HJ, Moir JL, Cameron KC, Clough TJ, Watson CJ, Grant J, Somers C, Richards KG (2015) Confirmation of co-denitrification in grazed grassland. Sci Rep 5:17361. https://doi.org/10.1038/srep17361
Senbayram M, Chen R, Budai A, Bakken L, Dittert K (2012) N2O emission and the N2O/(N2O + N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric Ecosyst Environ 147:4–12. https://doi.org/10.1016/j.agee.2011.06.022
Senbayram M, Wei Z, Wu D, Shan J, Yan X, Well R (2022) Inhibitory effect of high nitrate on N2O reduction is offset by long moist spells in heavily N loaded arable soils. Biol Fertil Soils 58:77–90. https://doi.org/10.1007/s00374-021-01612-x
Shoun H, Kim D-H, Uchiyama H, Sugiyama J (1992) Denitrification by fungi. FEMS Microbiol Lett 94:277–281
Simpson SL, Fitzpatrick RW, Shand P, Angel BM, Spadaro DA, Mosley L (2010) Climate-driven mobilisation of acid and metals from acid sulfate soils. Mar Freshw Res 61:129–138. https://doi.org/10.1071/MF09066
Spott O, Russow R, Stange CF (2011) Formation of hybrid N2O and hybrid N2 due to codenitrification: first review of a barely considered process of microbially mediated N-nitrosation. Soil Biol Biochem 43:1995–2011. https://doi.org/10.1016/j.soilbio.2011.06.014
Spott O, Stange F (2011) Formation of hybrid N2O in a suspended soil due to co-denitrification of NH2OH. J Plant Nutr Soil Sci 174:554–567. https://doi.org/10.1002/jpln.201000200
Stein LY (2019) Insights into the physiology of ammonia-oxidizing microorganisms. Curr Opin Chem Biol 49:9–15. https://doi.org/10.1016/j.cbpa.2018.09.003
Stevens RJ, Laughlin RJ (2001) Lowering the detection limit for dinitrogen using the enrichment of nitrous oxide. Soil Biol Biochem 33:1287–1289. https://doi.org/10.1016/S0038-0717(01)00036-0
Stevens RJ, Laughlin RJ, Atkins GJ, Prosser SJ (1993) Automated determination of nitrogen-15-labeled dinitrogen and nitrous oxide by mass spectrometry. Soil Sci Soc Am J 57:981–988. https://doi.org/10.2136/sssaj1993.03615995005700040017x
Stevens RJ, Laughlin RJ, Malone JP (1998) Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol Biochem 30:1119–1126
Takeda N, Friedl J, Rowlings D, De Rosa D, Scheer C, Grace P (2021) Exponential response of nitrous oxide (N2O) emissions to increasing nitrogen fertiliser rates in a tropical sugarcane cropping system. Agric Ecosyst Environ 313:107376. https://doi.org/10.1016/j.agee.2021.107376
Tanimoto T, Hatano K-i, Kim D-h, Uchiyama H, Shoun H (1992) Co-denitrification by the denitrifying system of the fungus Fusarium oxysporum. FEMS Microbiol Lett 93:177–180. https://doi.org/10.1016/0378-1097(92)90525-S
Tian H, Xu R, Canadell JG, Thompson RL, Winiwarter W, Suntharalingam P, Davidson EA, Ciais P, Jackson RB, Janssens-Maenhout G, Prather MJ, Regnier P, Pan N, Pan S, Peters GP, Shi H, Tubiello FN, Zaehle S, Zhou F et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256. https://doi.org/10.1038/s41586-020-2780-0
Van Breemen N (1982) Genesis, morphology, and classification of acid sulfate soils in coastal plains. In: Kittrick JA, Fanning DS, Hossner LR (eds) Acid sulfate weathering. SSSA Special Publications, pp 95–108. https://doi.org/10.2136/sssaspecpub10.c6
Van Cleemput O, Samater AH (1995) Nitrite in soils: accumulation and role in the formation of gaseous N compounds. Fert Res 45:81–89. https://doi.org/10.1007/BF00749884
Van den Heuvel RN, Bakker SE, Jetten MS, Hefting MM (2011) Decreased N2O reduction by low soil pH causes high N2O emissions in a riparian ecosystem. Geobiology 9:294–300. https://doi.org/10.1111/j.1472-4669.2011.00276.x
Wang X, Wells NS, Xiao W, Hamilton JL, Jones AM, Collins RN (2022) Mackinawite (FeS) Chemodenitrification of nitrate (NO3–) under acidic to neutral pH conditions and its stable N and O isotope dynamics. ACS Earth Space Chem 6:2801–2811. https://doi.org/10.1021/acsearthspacechem.2c00158
Warner DI, Scheer C, Friedl J, Rowlings DW, Brunk C, Grace PR (2019) Mobile continuous-flow isotope-ratio mass spectrometer system for automated measurements of N2 and N2O fluxes in fertilized cropping systems. Sci Rep 9:11097. https://doi.org/10.1038/s41598-019-47451-7
Weeg-Aerssens E, Tiedje JM, Averill BA (1988) Evidence from isotope labeling studies for a sequential mechanism for dissimilatory nitrite reduction. J Am Chem Soc 110:6851–6856. https://doi.org/10.1021/ja00228a039
Weier K, Rolston D, Thorburn P (1998) The potential for N losses via denitrification beneath a green cane trash blanket. Proc Australian Soc Sugar Cane Techologists 20:169–175
Well R, Burkart S, Giesemann A, Grosz B, Köster JR, Lewicka-Szczebak D (2019) Improvement of the 15N gas flux method for in situ measurement of soil denitrification and its product stoichiometry. Rapid Commun Mass Spectrom 33:437–448
Wilson SJ, Song B, Phillips RL (2021) Determining chemical factors controlling abiotic codenitrification. ACS Earth Space Chem 5:186–196. https://doi.org/10.1021/acsearthspacechem.0c00225
Wilson BP, White I, Melville MD (1999) Floodplain hydrology, acid discharge and change in water quality associated with a drained acid sulfate soil. Mar Freshw Res 50:149–157. https://doi.org/10.1071/MF98034
Wood S (2015) Package ‘mgcv’. R Package Version 1(29):729
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O (2018) The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3–A16. https://doi.org/10.1016/j.soilbio.2018.03.020
WRB IWg (2015) Working Group (2015) World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps FAO, Rome
Xu C, Wong VNL, Tuovinen A, Simojoki A (2023) Effects of liming on oxic and anoxic N2O and CO2 production in different horizons of boreal acid sulfate soil and non-acid soil under controlled conditions. Sci Total Environ 857:159505. https://doi.org/10.1016/j.scitotenv.2022.159505
Yli-Halla M, Virtanen S, Regina K, Österholm P, Ehnvall B, Uusi-Kämppä J (2020) Nitrogen stocks and flows in an acid sulfate soil. Environ Monit Assess 192:751. https://doi.org/10.1007/s10661-020-08697-1
Yoon S, Song B, Phillips RL, Chang J, Song MJ (2019) Ecological and physiological implications of nitrogen oxide reduction pathways on greenhouse gas emissions in agroecosystems. FEMS Microbiol Ecol 95:fiz066. https://doi.org/10.1093/femsec/fiz066
Zaman M, Kleineidam K, Bakken L, Berendt J, Bracken C, Butterbach-Bahl K, Cai Z, Chang SX, Clough T, Dawar K, Ding WX, Dörsch P, dos Reis MM, Eckhardt C, Fiedler S, Frosch T, Goopy J, Görres C-M, Gupta A et al (2021) Isotopic Techniques to Measure N2O, N2 and Their Sources. In: Zaman M, Heng L, Müller C (eds) Measuring emission of agricultural greenhouse gases and developing mitigation options using nuclear and related techniques: applications of nuclear techniques for GHGs. Springer International Publishing, Cham, pp 213–301. https://doi.org/10.1007/978-3-030-55396-8_7
Zhang C, Ju X, Zhang J, Rees RM, Müller C (2023) Soil pH and long-term fertilization affect gross N transformation and N2O production pathways in Chinese and UK croplands. Biol Fertil Soils 59:527–539. https://doi.org/10.1007/s00374-022-01695-0
Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB (2015) The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126:251–267. https://doi.org/10.1007/s10533-015-0166-4
Acknowledgements
We would like to thank Robert Quirk for granting us access to his property and Darcy Hayes for support with soil sampling. We would also like to thank Christian Brunk for his support with hardware and monitoring infrastructure, and Rene Diocares for his feedback regarding method development.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The data reported in this paper were partly obtained at the Central Analytical Research Facility (CARF) at Queensland University of Technology (QUT). Access to CARF is supported by generous funding from the Science Faculty (QUT). Johannes Friedl acknowledges support from the Austrian Science Fund through funding for the project ‘Sources matter - N2O production and consumption’ (ESP 319-B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kirkby, R., Friedl, J., De Rosa, D. et al. Hybrid pathways of denitrification drive N2O but not N2 emissions from an acid-sulphate sugarcane soil. Biol Fertil Soils (2024). https://doi.org/10.1007/s00374-023-01783-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00374-023-01783-9