Abstract
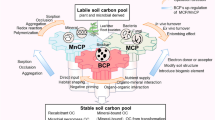
Microbial necromass accrual via anabolism is an important process contributing to the formation and accumulation of stable soil organic C (SOC). Both substrates and microbial community traits impact the rate and efficiency of microbial biomass production, yet their effects on necromass accumulation patterns and efficiency remain unclear. Here we selected six substrates to investigate substrate and community regulations on microbial necromass accumulation over a 476-day model soil incubation including three stages (microbial growth–starvation–reactivation) with varied supply of substrates or 13C-labeled glucose. Microbial respiration, the composition of main microbial groups, and necromass (amino sugars) production were examined along with 13C analysis to compare necromass accumulation from new OC and native SOC. We found that relative to fast-decomposing substrates, slow-decomposing substrate like lignin induced slow but steady necromass accrual in a long term. Exogenous glucose input stimulated necromass accumulation from new and native SOC during reactivation, especially in C (or energy)-limited model soils. Furthermore, the accumulation efficiency of amino sugars sourced from SOC and 13C-labeled glucose were positively related, suggesting microbial communities and soil properties affect necromass accrual efficiency. However, there was quite some scatter in the correlation, suggesting potential substrate effect and uncertainty in this relationship. Hence, the efficiency of soil “microbial C pump” (i.e., microbial transformation of exogenous substrates into microbial byproducts) fueled by exogenous substrate and native SOC needs to be further investigated. Collectively, these findings provide new information on the dynamics and efficiency of microbial necromass accumulation from different substrates in soils.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Adingo S, Yu J-R, Xuelu L, Li X, Jing S, Xiaong Z (2021) Variation of soil microbial carbon use efficiency (CUE) and its influence mechanism in the context of global environmental change: a review. PeerJ 9:e12131
Allison SD (2014) Modeling adaptation of carbon use efficiency in microbial communities. Front Microbiol 5:571
Appuhn A, Joergensen RG (2006) Microbial colonisation of roots as a function of plant species. Soil Biol Biochem 38:1040–1051
Bååth E, Anderson T-H (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Bai Z, Bodé S, Huygens D, Zhang X, Boeckx P (2013) Kinetics of amino sugar formation from organic residues of different quality. Soil Biol Biochem 57:814–821
Bailey VL, Peacock AD, Smith JL HB Jr (2002) Relationships between soil microbial biomass determined by chloroform fumigation–extraction, substrate-induced respiration, and phospholipid fatty acid analysis. Soil Biol Biochem 34:1385–1389
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brown RW, Chadwick DR, Bending GD, Collins CD, Whelton HL, Daulton E, Covington JA, Bull ID, Jones DL (2022) Nutrient (C, N and P) enrichment induces significant changes in the soil metabolite profile and microbial carbon partitioning. Soil Biol Biochem 172:108779
Buckeridge KM, Mason KE, Ostle N, McNamara NP, Grant HK, Whitaker J (2022) Microbial necromass carbon and nitrogen persistence are decoupled in agricultural grassland soils. Commun Earth Environ 3:114
Cai Y, Ma T, Wang Y, Jia J, Jia Y, Liang C, Feng X (2022) Assessing the accumulation efficiency of various microbial carbon components in soils of different minerals. Geoderma 407:115562
Chen J, Jia B, Gang S, Li Y, Li F-C, Mou XM, Kuzyakov Y, Li XG (2022) Decoupling of soil organic carbon and nutrient mineralization across plant communities as affected by microbial stoichiometry. Biol Fertil Soils 58:693–706
Chen Y, Neilson JW, Kushwaha P, Maier RM, Barberan A (2021) Life-history strategies of soil microbial communities in an arid ecosystem. ISME J 15:649–657
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19:988–995
Craig ME, Geyer KM, Beidler KV, Brzostek ER, Frey SD, Stuart Grandy A, Liang C, Phillips RP (2022) Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat Commun 13:1229
Cui J, Zhu Z, Xu X, Liu S, Jones DL, Kuzyakov Y, Shibistova O, Wu J, Ge T (2020) Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol Biochem 142:107720
Dendooven L, Alcántara-Hernández RJ, Valenzuela-Encinas C, Luna-Guido M, Perez-Guevara F, Marsch R (2010) Dynamics of carbon and nitrogen in an extreme alkaline saline soil: A review. Soil Biol Biochem 42:865–877
Deng S, Zheng X, Chen X, Zheng S, He X, Ge T, Kuzyakov Y, Wu J, Su Y, Hu Y (2021) Divergent mineralization of hydrophilic and hydrophobic organic substrates and their priming effect in soils depending on their preferential utilization by bacteria and fungi. Biol Fertil Soils 57:65–76
Ding GC, Pronk GJ, Babin D, Heuer H, Heister K, Kӧgel-Knabner I, Smalla K (2013) Mineral composition and charcoal determine the bacterial community structure in artificial soils. FEMS Microbiol Ecol 86:15–25
Engelking B, Flessa H, Joergensen RG (2007) Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol Biochem 39:2111–2118
Feng X, Simpson MJ (2009) Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol Biochem 41:804–812
Forster JC (1995) Soil sampling, handling, storage and analysis. In: Alef K, Nannapieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press Inc, San Diego, pp 49–121
Glaser B, Turrión M-B, Alef K (2004) Amino sugars and muramic acid-biomarkers for soil microbial community structure analysis. Soil Biol Biochem 36:399–407
Guckert JB, Antworth CP, Nichols PD, White DC (1985) Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microbiol Ecol 31:147–158
Guerrant GO, Moss CW (1984) Determination of monosaccharides as aldononitrile, O-methyloxime, alditol, and cyclitol acetate derivatives by gas chromatography. Anal Chem 56:633–638
Gunina A, Dippold MA, Glaser B, Kuzyakov Y (2014) Fate of low molecular weight organic substances in an arable soil: from microbial uptake to utilisation and stabilisation. Soil Biol Biochem 77:304–313
Gunina A, Kuzyakov Y (2022) From energy to (soil organic) matter. Glob Chang Biol 28:2169–2182
Harris DJ, Horwath WR, Van Kessel C (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci Soc Am J 65:1853–1856
Hill PW, Farrar JF, Jones DL (2008) Decoupling of microbial glucose uptake and mineralization in soil. Soil Biol Biochem 40:616–624
Huang X, Liu S, Wang H, Hu Z, Li Z, You Y (2014) Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol Biochem 73:42–48
Islam KR, Weil RR, Mulchi CL, Glenn SD (1997) Freeze-dried soil extraction method for the measurement of microbial biomass C. Biol Fertil Soils 24:205–210
Jagadamma S, Mayes MA, Phillips JR (2012) Selective sorption of dissolved organic carbon compounds by temperate soils. PLoS One 7:e50434
Jia J, Feng X, He J-S, He H, Lin L, Liu Z (2017) Comparing microbial carbon sequestration and priming in the subsoil versus topsoil of a Qinghai-Tibetan alpine grassland. Soil Biol Biochem 104:141–151
Joergensen RG (2018) Amino sugars as specific indices for fungal and bacterial residues in soil. Biol Fertil Soils 54:559–568
Joergensen RG (2022) Phospholipid fatty acids in soil—drawbacks and future prospects. Biol Fertil Soils 58:1–6
Jones DL, Hill PW, Smith AR, Farrell M, Ge T, Banning NC, Murphy DV (2018) Role of substrate supply on microbial carbon use efficiency and its role in interpreting soil microbial community-level physiological profiles (CLPP). Soil Biol Biochem 123:1–6
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630
Kästner M, Miltner A, Thiele-Bruhn S, Liang C (2021) Microbial necromass in soils—linking microbes to soil processes and carbon turnover. Front Environ Sci 9:756378
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems - a review. Plant Soil 256:41–66
LaRowe DE, Van Cappellen P (2011) Degradation of natural organic matter: a thermodynamic analysis. Geochim Cosmochim Acta 75:2030–2042
Liang C, Amelung W, Lehmann J, Kastner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Chang Biol 25:3578–3590
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105
Liu X, Hou Y, Yu Z, Wang Y, Zhou S, Jiang B, Liao Y (2020) Comparison of molecular transformation of dissolved organic matter in vermicomposting and thermophilic composting by ESI-FT-ICR-MS. Environ Sci Pollut Res 27:43480–43492
Manzoni S, Čapek P, Porada P, Thurner M, Winterdahl M, Beer C, Brüchert V, Frouz J, Herrmann AM, Lindahl BD, Lyon SW, Šantrůčková H, Vico G, Way D (2018) Reviews and syntheses: carbon use efficiency from organisms to ecosystems - definitions, theories, and empirical evidence. Biogeosciences 15:5929–5949
Manzoni S, Taylor P, Richter A, Porporato A, Agren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91
Martin JP, Haider K, Linhares LF (1979) Decomposition and stabilization of ring-14C-labeled catechol in soil. Soil Sci Soc Am J 43:100–104
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55
Miyamoto-Shinohara Y, Imaizumi T, Sukenobe J, Murakami Y, Kawamura S, Komatsu Y (2000) Survival rate of microbes after freeze-drying and long-term storage. Cryobiology 41:251–255
Miyamoto-Shinohara Y, Sukenobe J, Imaizumi T, Nakahara T (2006) Survival curves for microbial species stored by freeze-drying. Cryobiology 52:27–32
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Olagoke FK, Bettermann A, Nguyen PTB, Redmile-Gordon M, Babin D, Smalla K, Nesme J, Sørensen SJ, Kalbitz K, Vogel C (2022) Importance of substrate quality and clay content on microbial extracellular polymeric substances production and aggregate stability in soils. Biol Fertil Soils 58:435–457
Pinnataip R, Lee BP (2021) Oxidation chemistry of catechol utilized in designing stimuli-responsive adhesives and antipathogenic biomaterials. ACS Omega 6:5113–5118
R Development Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing. R Development Core Team, Vienna
Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J, Peck MW, Hinton JC (2012) Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol 194:686–701
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol 3:348
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16:930–939
Soares M, Rousk J (2019) Microbial growth and carbon use efficiency in soil: Links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol Biochem 131:195–205
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53
Wickland KP, Neff JC, Aiken GR (2007) Dissolved organic carbon in Alaskan boreal forest: sources, chemical characteristics, and biodegradability. Ecosystems 10:1323–1340
Wild B, Schnecker J, Alves RJ, Barsukov P, Barta J, Capek P, Gentsch N, Gittel A, Guggenberger G, Lashchinskiy N, Mikutta R, Rusalimova O, Santruckova H, Shibistova O, Urich T, Watzka M, Zrazhevskaya G, Richter A (2014) Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol Biochem 75:143–151
Xu X, Schimel JP, Thornton PE, Song X, Yuan F, Goswami S (2014) Substrate and environmental controls on microbial assimilation of soil organic carbon: a framework for Earth system models. Ecol Lett 17:547–555
Xu Y, Gao X, Liu Y, Li S, Liang C, Lal R, Wang J (2022) Differential accumulation patterns of microbial necromass induced by maize root vs. shoot residue addition in agricultural Alfisols. Soil Biol Biochem 164:108474
Xu Y, Seshadri B, Sarkar B, Wang H, Rumpel C, Sparks D, Farrell M, Hall T, Yang X, Bolan N (2018) Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci Total Environ 621:148–159
Zhang X, Amelung W (1996) Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol Biochem 28:1201–1206
Zhou C, Liu Y, Liu C, Liu Y, Tfaily MM (2019) Compositional changes of dissolved organic carbon during its dynamic desorption from hyporheic zone sediments. Sci Total Environ 658:16–23
Zhu E, Cao Z, Jia J, Liu C, Zhang Z, Wang H, Dai G, He JS, Feng X (2021) Inactive and inefficient: warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob Chang Biol 27:2241–2253
Acknowledgements
We thank the Plant Science Facility of the Institute of Botany, Chinese Academy of Sciences for bulk soil analysis.
Funding
This study was financially supported by the National Key R&D Program of China (2019YFA0607303), the National Natural Science Foundation of China (42103028), and the Chinese Academy of Sciences K. C. Wong Education Foundation (GJTD-2019-10).
Author information
Authors and Affiliations
Contributions
Y. C. and X. F. conceived the idea and designed the study. Y. C. conducted the experiment and analyzed the data; Y. C. and X. F. wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Supplementary information related to this article can be found in the online version of this article. (DOCX 537 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Y., Feng, X. Substrate and community regulations on microbial necromass accumulation from newly added and native soil carbon. Biol Fertil Soils 59, 763–775 (2023). https://doi.org/10.1007/s00374-023-01745-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-023-01745-1