Abstract
This study investigated the effects of irrigation frequency on N2 and N2O emissions from an intensively managed pasture in the subtropics. Irrigation volumes were estimated to replace evapotranspiration and were applied either once (low frequency) or split into four applications (high frequency). To test for legacy effects, a large rainfall event was simulated at the end of the experiment. Over 15 days, 7.9 ± 2.7 kg N2 + N2O-N ha−1 was emitted on average regardless of irrigation frequency, with N2O accounting for 25% of overall N2 + N2O. Repeated, small amounts of irrigation produced an equal amount of N2 + N2O losses as a single, large irrigation event. The increase in N2O emissions after the large rainfall event was smaller in the high-frequency treatment, shifting the N2O/(N2O + N2) ratio towards N2, indicating a treatment legacy effect. Cumulative losses of N2O and N2 did not differ between treatments, but higher CO2 emissions were observed in the high-frequency treatment. Our results suggest that the increase in microbial activity and related O2 consumption in response to small and repeated wetting events can offset the effects of increased soil gas diffusivity on denitrification, explaining the lack of treatment effect on cumulative N2O and N2 emissions and the abundance of N cycling marker genes. The observed legacy effect may be linked to increased mineralisation and subsequent increased dissolved organic carbon availability, suggesting that increased irrigation frequency can reduce the environmental impact (N2O), but not overall magnitude of N2O and N2 emissions from intensively managed pastures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetting and drying cycles lead to rapid changes in soil moisture in pasture soils, triggering pulses of nitrous oxide (N2O) and, if the microbial process of denitrification goes to completion, dinitrogen (N2) emissions (Friedl et al. 2017). Emissions of N2 and N2O are governed by complex feedbacks between nitrogen (N) and carbon (C) substrate availability (Azam et al. 2002; Giles et al. 2012), temperature (Blagodatskaya et al. 2014; Stres et al. 2008), soil pH (Baggs et al. 2010; Čuhel and Šimek 2011), soil oxygen (O2) status (Rohe et al. 2021) and the physiological response of the soil microbial community to these factors (Kuypers et al. 2018). Soil moisture exerts an overarching control, as it determines substrate diffusion, but also diffusion of gases into and within the soil matrix (Blagodatsky and Smith 2012), and thus the production and the movement of N2O and N2 in the soil, defining temporal variability, overall magnitude and N2O:N2 partitioning of resulting N2O and N2 surface emissions. The N2O:N2 ratio defines the environmental impact of these emissions, since N2O is a potent greenhouse gas, and the single most ozone-depleting substance in the stratosphere (Ravishankara et al. 2009). Shifts in amplitude and frequency of wetting and drying cycles under current climate change scenarios, but also changes in irrigation schemes are likely to cause shifts in microbial function (Evans and Wallenstein 2012), in microbial activity (Banerjee et al. 2016) and ultimately in production of N2O in pasture soils. The general lack of in situ measurements of N2 however hinders accurate predictions how the role of pasture soils as sources and sinks of N2O will change under the predicted changes of wetting and drying cycles.
Enzyme activity related to N cycling is tightly linked to O2 availability in soils: The rate limiting step of nitrification, the oxidation of the NH3 to hydroxylamine by the ammonia mono-oxygenase (AMO) requires O2 and is therefore inhibited under anaerobic conditions. Classic heterotrophic denitrifiers (Braker et al. 2012), but also other organisms capable of denitrification (Wrage-Mönnig et al. 2018) switch to NO3− or nitrite (NO2−) as electron acceptors if O2 becomes limiting, producing variable amounts of nitric oxide (NO), N2O and N2. The activities of the relative enzymes, i.e. the NO2− reductase (NIRS), the NO reductase (NORB) and the N2O reductase (NOS), are negatively correlated to O2 availability, with NOS being the most sensitive to O2 inhibition (Morley et al. 2008).
Relative soil gas diffusivity (DP/DO) is defined by the soil gas diffusion coefficient DP (cm3 air cm−1 soil s−1) and the gas diffusion coefficient in free air DO (cm2 air s−1) and describes potential O2 diffusion through and into the soil, accounting for the interaction between bulk density, pore size distribution and soil water content (Moldrup et al. 2013). In contrast to soil water-filled pore space (WFPS), DP/DO enables comparisons between soils with differing bulk densities (Farquharson and Baldock 2008). The onset of anaerobic conditions in the soil has been reported for DP/DO < 0.02 (Stepniewski 1981), while decreasing N2O emissions due to increased reduction to N2 were observed at DP/DO < 0.006 (Balaine et al. 2016). The negative correlation between DP/DO and N2O (Clough et al. 2020) suggests that wetting and drying cycles that maximise DP/DO will minimise the formation of anaerobiosis in soils, limiting N2O and N2 emissions. This hypothesis has been supported by irrigation studies in cropping (Jamali et al. 2015) and pasture (Rousset et al. 2021) systems, where the split application of irrigation increased soil aeration and thus reduced N2O emissions. However, soil O2 concentration is defined by the balance of supply and consumption (Rohe et al. 2021), and N2 and N2O emissions well above the postulated DP/DO threshold have been attributed to increased microbial O2 consumption driven by easily available C in pasture soils (Friedl et al. 2021; Petersen et al. 2013). Both O2 consumption and reduced DP/DO will ultimately determine magnitude and partitioning of N2O and N2 emissions, yet their significance is likely to shift as the amplitude and frequency of wetting and drying cycles changes.
This study is part of a trial on an intensively managed dairy pasture, where effects of increased irrigation frequency, that is more frequent and small irrigation events versus large and infrequent events, on N cycling and loss were investigated. Previous work at the site (Mumford et al. 2019) demonstrated no effects of increased irrigation frequency on pasture yield or plant N uptake, but a reduction of N2O emissions. This reduction was not directly linked to irrigation events but to a treatment legacy observed after intense rainfall.
The study presented here investigated the underlying mechanisms explaining the observed reduction of N2O emissions, linking in situ measurements of N2O and N2 from an intensively managed dairy pasture to DP/DO, C and N substrate availability and the abundance of functional marker genes for N cycling. The experimental setup not only enabled to establish the response of N2O to increased irrigation frequency, but also allowed to demonstrate shifts in the N2O:N2 ratio, defining the environmental impact of these emissions. We hypothesised that a) increased irrigation frequency would reduce overall N2 and N2O losses and shift the N2O/(N2O + N2) ratio towards N2 and b) that the treatment legacy after large rainfall was linked to reduced NO3− availability and a subsequent decrease in the N2O/(N2O + N2) ratio. The first study to establish the response of N2O and N2 emissions to different wetting and drying cycles from subtropical pasture soils will help to improve our quantitative process understanding for N2O and N2 production following irrigation, and under changing precipitation patterns.
Materials and methods
The study was conducted on a commercial dairy farm in Casino, New South Wales (28.865°S, 152.874°E). Site characteristics including soil physical and chemical parameters are shown in Table 1. The climate at the site is humid subtropical with summer-dominated rainfall. Rainfall variation at the site can be extremely large with monthly totals in excess of 400 mm common. Mean annual precipitation is 1037 mm, and average daily temperature ranges from 18 °C to 30 °C in summer and 6.6 °C to 22 °C during winter. The soil at the site is a black Vertosol (Isbell 2016), which corresponds to a Pellic Vertisol (IUSS 2015), with clay content increasing with depth. The average farm stocking rate is 5 heads of cattle ha−1, supported by average fertiliser inputs of 340 kg N ha−1 year−1. On average, 210 head of cattle graze an area of 1.2 ha−1 over 12 h every 14–21 days (De Rosa et al. 2020). Fields are usually grazed and fertilised on a 2-to-3-week cycle from the beginning of May until mid-November, with irrigation applied after fertilisation. Fertiliser N rates range from 1 to 2 kg N ha−1 day−1 applied as urea (Mumford et al. 2019). The summer dominant Kikuyu pasture (Pennisetum clandestinum) is usually mulched at the end of April and oversown with annual ryegrass (Lolium italicum).
Experimental setup
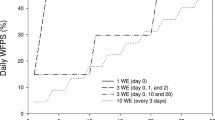
The study was conducted as part of a trial investigating the effects of wetting and drying cycles on N cycling in intensively managed pastures (Mumford et al. 2019). Wetting and drying cycles were simulated using a remote-controlled irrigation system replacing the estimated evapotranspiration (ET) rate by applying the respective amount of irrigation at once (low frequency—LF) or split into 4 irrigation events (high frequency—HF) over a grazing cycle (Fig. 1). Evapotranspiration was estimated based on climate data from the 3 previous years and adjusted to crop ET in mm day−1 using the respective crop coefficient for ryegrass (Allen et al. 1998). Irrigation was applied with sprinklers at a rate of 0.32 mm minute−1. The LF and HF treatments with four replicate plots of 25 m2 were established in a randomised plot design with a 1 m buffer between plots in June 2017 after ryegrass establishment (Figure S1), matching the treatments allocation from previous studies. The treatment history is shown in Table S1.
Outline of the experiment showing grazing, N fertilisation and the application of the high (light blue) and low frequency (dark blue) irrigation treatments and the simulated rainfall event (black) as a percentage of estimated evapotranspiration for 6 cycles of irrigation history and during the time of the experiment. Resulting water-filled pore space (WFPS) is shown for the experimental period
Grazing was simulated by mowing the plots to 5 cm and clippings were removed, protecting monitoring equipment in place. Urea N fertiliser was applied after grazing, followed by an irrigation event (HF or LF) (Fig. 1). In the beginning of the ryegrass season, rainfall exclusion shelters (3.6 * 1.5 m) were established on the plots and opened for each irrigation event. Two steel bases (i.e. microplots) were installed under each shelter 3 weeks before the start of the experiment: A 0.5 by 0.5 m base for soil sampling; and one 0.18 m by 0.18 m base for N2 and N2O measurements, all remaining in situ for the length of the experiment. All plots were mowed and fertilised with urea at a rate of 32 kg N ha−1 on 31 October 2017. Fertiliser N was applied as a urea solution equivalent to 2 mm irrigation a) at 15N natural abundance on the soil sampling microplot, b) at 98% 15N atom excess on the N2 + N2O microplot and c) the rest of the plot received granular urea at the same rate. The HF treatment received 21.4 mm on day 0, 5 and 8 after fertilisation, while the LF treatment received 86.6 mm after fertilisation. To test for legacy effects of irrigation frequency on N2O and N2 emissions, a rainfall event of 100 mm was simulated across treatments saturating to top 10 cm of the soil. This was done 10 days after fertilisation, when soil WFPS was similar in both treatments (Figs. 1, 2) to exclude effects of antecedent soil moisture on N2O emissions.
Daily emissions of N2O and N2, cumulative emissions of N2O + N2 (g N ha−1 day−1), soil WFPS, soil gas diffusivity (DP/DO) from an intensively managed dairy pasture in response to high frequency (HF) irrigation, low frequency (LF) irrigation and a simulated rainfall event. The continuous line in the WFPS figure shows field capacity. The continuous line in the DP/DO graph marks the proposed threshold for the formation of anaerobic sites in the soil matrix (Stepniewski 1981) and the dashed line shows the proposed threshold for maximum N2O production (Balaine et al. 2016)
Gas sampling and analysis
The static closed chamber method was used to measure N2, N2O and CO2 emissions. Manual gas samples were taken at day 1, 2, 3, 4, 5, 6, 9, 10, 11, 12, 13 and 15 days after fertilisation, with samples taken between 9 and 12 am. Polyethylene chambers with a headspace height of 31.4 cm for the N2 microplots were placed on the steel frames, and headspace gas samples (20 ml) were taken by connecting a syringe to a 2-way Luer-Lock tap installed in the lid of the chamber 0, 60 and 180 min after chamber closure. Gas samples were injected into a pre-evacuated 12 ml glass vial with a double wadded Teflon/silicon septa cap (Labco, UK). Temperature inside the chambers was recorded with a temperature logger (HOBO onset UA-002–64).
Headspace gas samples were analysed for N2O and CO2 by gas chromatography (GC) (Shimadzu GC-2014), and for the isotopologues of N2 (15N14N, 15N15N) and N2O ([14N15N16O + 15N14N16O] and 15N15N16O) using an automated isotope ratio mass spectrometer (IRMS) coupled to a trace gas preparation unit (Sercon Limited, 20–20, UK).
Aboveground biomass sampling and analysis
At the end of the experiment, 2 biomass cuts at grazing height (> 5 cm) from random positions under the rain shelter were bulked from each plot using a 0.5 m × 0.5 m metal quadrat. Plant material was oven dried at 60 °C, ground with a planetary cylinder mill and analysed for N content using a LECO TruMac CNS analyser (MI, USA).
Soil sampling
Destructive soil samples were taken from the soil sampling microplots 1 day prior, and 2, 4, 6, 10, 12, 13 and 15 days after fertilisation using a soil corer with an inner diameter of 17 mm. For soil mineral N, dissolved organic N (DON) and C (DOC) analysis, 3 bulk samples (0–10 cm) from the topsoil were taken from each microplot, bulked together and stored at 5 °C at the site. For DNA extraction, 3 bulk samples (0–10 cm) were homogenised and separated from the root biomass on site. Aliquots of 250 mg were directly suspended in extraction buffer and stored at 5 °C. Both bulk soil samples and soil in extraction buffer were transported cooled to the laboratory within a day of sampling.
Soil mineral N, DON and DOC
Soil samples were extracted with a 2 M KCl solution (1:5 w:v) for 1 h on a rotary shaker and filtered through Whatman No. 42 filter paper. The concentration of exchangeable NH4+-N and NO3–-N (measured as a sum parameter of NO3− + NO2−) in the KCl extracts was quantified with a Gallery™ Discrete Analyzer (Thermo Fisher Scientific). Soil was extracted for DON and DOC with deionised water (1:5 w:v) for 1 h on a rotary shaker, centrifuged (27,179 g, 30 min, 4 °C), filtered through a 0.45 µm membrane filter and analysed by Shimadzu TOC-TN analyser (Shimadzu Corporation, Kyoto, Japan). Soil DON concentrations were calculated as the difference between total N in the water extract and mineral N concentrations.
Extraction of DNA and quantitative polymerase chain reaction (qPCR) of functional marker genes
Soil DNA was extracted using the PowerSoil® DNA Isolation Kit MoBio (Mobio Laboratories, Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions. Concentration of DNA and quality were determined spectrophotometrically (NanoDrop 2000, Thermo Fisher, MA, USA). Primer sets used for the quantification of functional marker genes of nitrification (amoA AOA and amoA AOB) and denitrification (nirS and nosZ) are listed in Table S2. The qPCR assay was carried out in a volume of 10 µL, and the assay mixture contained Phusion™ High-Fidelity DNA Polymerase, 10 µM of each Primer (except for the gene amoA AOA, where 20 µM was used) and 1 µL of the template DNA. Each sample was pipetted in triplicates using a robot (Eppendorf epMotion 5075t, Eppendorf AG, Hamburg, Germany) and quantified using the CFX384 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Standard curves were constructed as described in Friedl et al. (2020b).
Auxiliary measurements
Soil water content of the HF and LF treatment was logged in 30-min intervals using domain reflectance probes (SentekTable 2 EnviroSCAN, South Australia) at 5 cm depth. Raw outputs were calibrated using soil water characteristics determined using intact soil cores from the site on a pressure plate apparatus.
Calculations and statistical analysis
Emissions of N2O, CO2 and N2
The slope of the linear increase or decrease in N2O and CO2 concentration during the chamber closure was used to calculate GHG fluxes, corrected for air temperature, atmospheric pressure and the ratio of chamber volume to the surface area. The coefficient of determination (R2) for linear regression was used as a quality check and flux rates were discarded if R2 was < 0.80 for N2O and < 0.95 for CO2. Fluxes of N2 were calculated assuming a linear increase in soil-derived N2 in the chamber headspace.
The ion currents (I) at m/z 44, 45 and 46 enabled the molecular ratios 45R (45I/44I) and 46R (46I/44I) to be calculated for N2O, and I at m/z 28, 29 and 30 enabled 29R (28I/29I) and 30R (28I/30I) to be calculated for N2. The 15N enrichment of the NO3− pool undergoing denitrification (ap N2 and ap N2O) and the fraction of N2 and N2O emitted from this pool (fp) were calculated following the equations given by Spott et al. (2006):
where abgd is the 15 N abundance of the atmospheric background and am is the measured 15 N abundance of N2 or N2O, calculated as
The 15 N enrichment of the soil NO3− pool undergoing denitrification ap is calculated for N2 and N2O as
To calculate ap N2O, 45R and 46R were converted to 29R and 30R by correcting for the naturally occurring O2 isotopes:
using the value of 0.00038 for 17R and 0.002079 for 18R (Arah 1997).
The measured fraction of m/z 30 in N2 and converted N2O 30xm is calculated as:
Assuming that N2 and N2O originate from the same NO3− pool undergoing denitrification, ap derived from N2O was used to calculate N2 fluxes. If only 29R was > the detection limit (DL), fp was calculated as
Formula 7 was used to calculate 60% of all N2 fluxes. The headspace concentrations of N2O and N2 were multiplied by the respective fp values giving N2 and N2O produced via denitrification (referred to as N2 and N2Od), with their respective fluxes expressed in g N2 or N2Od –N emitted g−1 soil day−1.
The between batch precision of the IRMS for N2 based on the standard deviation of atmospheric air samples (n = 36) at 95% confidence intervals (Friedl et al. 2020a) was 4.4 × 10–7 and 6.61 × 10–7 for 29R and 30R, respectively. The corresponding method detection limit ranged from 34 g N2-N ha−1 day−1 with ap assumed at 60 atom % to 303 g N2-N ha−1 day−1 with ap assumed at 20 atom %.
Soil gas diffusivity DP/DO was calculated for 0–10 cm soil depth using the structure-dependent, water-induced linear reduction model of Moldrup et al. (2013) with the media complexity factor of 2.1 for intact soil.
The effect of irrigation frequency on emissions of N2, N2O, CO2 and the abundance of nosZ and nirS was assessed using linear mixed-effect models (R Package “nlme”) (Pinheiro et al. 2019).
Relative changes of the N2O/(N2 + N2O) ratio and substrate availability for denitrification (DOC, NO3−) in response to the rainfall event were calculated as the difference in the plot specific values prior and after rainfall. The effect of irrigation frequency on relative changes of the N2O/(N2 + N2O) ratio and substrate availability for denitrification (NO3−, DOC), was analysed by analysis of variance (ANOVA) (P < 0.05). Values in the text, tables and figures represent means ± standard error of the mean.
Results
Figure 2 shows the response of soil water content, soil gas diffusivity (DP/DO) and emissions of N2 and N2O to different wetting and drying cycles (HF and LF) and the rainfall event.
Soil water and soil gas diffusivity
Actual ET at the site was 75.7 mm, 13% lower than ET estimated based on the average of the previous 3 years (86.6 mm). The application of 86.6 mm of irrigation after fertilisation to the LF plots increased soil WFPS > 75%, and respective values for DP/DO decreased below 0.006 for 1.5 days. Soil DP/DO increased thereafter but remained below 0.02 until day 10. On the HF plots, 21.4 mm of irrigation increased soil WFPS from 51 to 58%. Corresponding values for DP/DO fell below 0.02 for 1 day and increased thereafter. Irrigation events on day 4 and 8 lowered DP/DO below 0.02 for 1 and 2 days, respectively. The soil water content on day 10 was 53 and 51% WFPS for the HF and the LF, respectively. The simulation of a 100 mm rainfall event at day 10 increased soil WFPS > 90%, decreasing DP/DO below 0.006 for 1.5 days in both treatments. Soil water content decreased until day 15 and values for DP/DO remained below 0.02. Regardless of treatment, WFPS remained above field capacity (54%) except for day 8 after fertilisation in HF.
Emissions of N2O and N2
Fluxes of N2O on day 1 after fertilisation ranged from 125 to 330 g N2O-N ha−1 day−1 (Fig. 2). Values for average daily N2O fluxes were higher from LF as compared to HF for the first 3 days after fertilisation, but differences were not significant. Irrigation on day 4 in HF increased N2O emissions > 100 g N2O-N ha−1 day−1, while respective fluxes from LF remained below 50 g N2O-N ha−1 day−1. The simulated rainfall event led to a pulse of N2O emissions for a day in both treatments, with average daily fluxes of 743 ± 33 and 1454 ± 692 g N2O-N ha−1 day−1 for the HF and LF, respectively. The variability between replicates was high, with N2O fluxes ranging from 250 to 1710 g N2O-N ha−1 day−1 for HF, and from 472 to 3502 g N2O-N ha−1 day−1 for LF. Subsequent N2O fluxes decreased to < 100 g N2O-N ha−1 day−1.
Emissions of N2 from LF on the first day after fertilisation were > 400 g N2-N ha−1 day−1 and exceeded N2 emissions from the HF by a factor of 2 for the first 2 days. The irrigation event on day 4 increased N2 emissions from the HF treatment > 300 g N2-N ha−1 day−1. The simulated rainfall event led to a sharp increase in N2 emissions, with 1140 ± 312 and 1737 ± 475 g N2-N ha−1 day−1 emitted from HF and LF, respectively. Fluxes of N2 decreased in both treatments until day 13. On day 15, N2 emissions > 1000 g N2-N ha−1 day−1 were observed from both treatments.
Regression analysis (Fig. 3) showed that the log of daily N2O-N and N2-N followed a linear relationship with log DP/DO (R2 = 0.72 and R2 = 0.43, respectively; P < 0.01) in LF. In HF, this relationship was not observed for N2O and log DP/DO explained only 1% ( R2 = 0.01) of the variation in log N2.
Cumulative N2 + N2O losses from the LF treatment exceeded those from HF for the first 5 days (P < 0.05). Over 15 days, 7302 ± 1476 and 8593 ± 1334 g N2 + N2O-N ha−1 were emitted from HF and LF, respectively, with no differences between treatments. Emissions of N2O accounted for 22 and 25% of overall N2 + N2O losses from HF and LF, respectively. Emissions of N2O and the N2O/(N2O + N2) ratio did not differ between treatments before the rainfall event. After rainfall, emissions of N2O increased in both treatments and exhibited high variability within treatments. The relative increase in N2O from day 10 to 11 was higher in LF, exceeding the increase observed for HF by a factor of 4 (P < 0.05). The proportion of N2 + N2O emissions lost as N2O increased from day 10 to day 11 from 4 to 37% for LF, and from 19 to 37% for HF. This shift in the N2O/(N2O + N2) ratio was significantly higher in LF as compared to HF (P < 0.05).
The enrichment of the NO3− pool undergoing denitrification (ap N2 and ap N2O) is plotted in Figure S2, showing a decrease in 15NO3− enrichment over time in both treatments over the time of the experiment. Differences between ap N2 and ap N2O were not statistically significant, supporting the use of ap N2O for N2 flux calculations. However, average values for ap N2 were below ap N2O on some days in the first half of the experiment.
Emissions of CO2
The differential of CO2 emissions (Δ CO2-C kg ha-1 day-1) calculated as the difference between CO2 emissions from HF and LF (Fig. 4) was used as an indicator of relative differences in heterotrophic soil respiration between treatments, as different wetting and drying cycles had no effect on pasture yield. Relative to LF, HF increased CO2 emissions (P < 0.05), with marked differences after irrigation event 2 and 3.
Soil mineral N, DON and DOC
Soil mineral N and DON concentrations did not differ between treatments (Fig. 5). Exchangeable soil NH4+ concentrations remained stable over the time of the experiment, ranging from 12 to 19 mg NH4+-N kg−1 soil. Soil NO3− concentrations increased after fertilisation and peaked at day 5 in both treatments at 19 to 22 mg NO3−-N kg−1 soil and subsequently decreased to 5 mg NO3−-N kg−1 at the end of the experiment. Soil DON and DOC increased over time and peaked at day 12 of the experiment, decreasing thereafter. Soil DON ranged from 38 to 100 mg DON-N kg−1 soil and did not differ between treatments. Soil DOC ranged from 451 to 1245 mg DOC-C kg−1 soil. The rainfall event increased DOC concentrations in HF by a factor of 1.4 from day 10 to 12 (P < 0.05).
Aboveground biomass
Aboveground biomass (> 5 cm) yield, N content in the aboveground biomass, and N yield did not respond to the irrigation treatments. Aboveground biomass yield was low at the end of the ryegrass season, with DM yields ranging from 0.74 ± 0.29 to 0.77 ± 0.29 t DM ha−1 for LF and HF, respectively. With an N content of 4.12 ± 0.47% and 4.72 ± 0.37%, N yield was 31.17 ± 12.66 kg N ha−1 for LF and HF, respectively.
Abundance of N cycling functional marker genes
The abundance of ammonia oxidising archaea (AOA) and bacteria (AOB) was quantified determining archaeal and bacterial amoA gene copy numbers. Archaeal gene copy numbers fluctuated between 1.7 × 107 and 6.8 × 107 g−1 dry soil and exceeded bacterial amoA gene copy numbers by around an order of magnitude (Fig. 6). The abundance of nirS as a proxy for functional genes involved in NO2− reduction ranged from 3.5 × 106 to 1.6 × 106 g−1 dry soil. Gene copy numbers of nosZ ranged from 5.0 × 107 to 1.7 × 107 g−1 dry soil, around an order of magnitude below the nirS copy numbers. The long-term simulation of different wetting and drying cycles had no significant effect on the abundance of functional marker genes quantified prior to fertilisation. The linear mixed-effect models did not show a significant effect of sampling date on the abundance of the investigated functional marker genes during the experiment, and thus no response to different wetting and drying cycles (HF and LF) and the rainfall event.
Discussion
Effect of irrigation frequencies on N2 and N2O emissions
Emissions of N2 exceeded N2O emissions by a factor of 4 over the time of the experiment. Peak N2 and N2O fluxes were consistent with spikes in soil WFPS causing declines in soil gas diffusivity (Dp/Do), suggesting increased anaerobicity promoting denitrification. The application of 15N labelled urea hinders N2O source partitioning (Arah 1997) between nitrification and denitrification, as the NH4+ pool cannot be assumed to be at natural 15N abundance. The relationship between soil water content and N2O emissions however suggests denitrification as the main pathway of N2O production; an assumption which is supported by results from an incubation study with the same pasture soil, demonstrating denitrification as the main source of N2O at WFPS ≥ 40% (Friedl et al. 2021). However, this study also showed significant contributions of nitrification mediated pathways to N2O production including autotrophic and heterotrophic nitrification, highlighting their potential significance for the observed N2O fluxes in the study presented here.
Differences in wetting and drying were reflected in the temporal response of DP/DO (Fig. 2): As expected, DP/DO was initially higher in the HF treatment. In the LF treatment, DP/DO fell below the proposed threshold of 0.006 (Balaine et al. 2016) after the initial irrigation of 84 mm, while DP/DO remained well above 0.006 in the HF treatment and dropped only below 0.02 (Stepniewski 1981) for short periods of time following repeated irrigation events of 21 mm. The comparison between treatments implies increased formation of anaerobic microsites in the LF treatment and therefore larger losses of N2 and N2O, consistent with the reported reduction of N2O from urine patches when maximising DP/DO (Rousset et al. 2021). However, cumulative N2 and N2O emissions after two irrigation events of 21 mm were as high as those observed after a single irrigation event of 84 mm, showing that the frequency of wetting events, and not just the absolute amount of soil water was driving the magnitude of N2 and N2O emissions. Pooling N2 and N2O emissions across DP/DO (Fig. 3) further highlights differences between treatments: The loglinear relationship between daily N2O-N and N2-N and DP/DO shows an exponential increase in both N2O and N2 with decreasing DP/DO, when emissions are triggered by single and large rainfall/irrigation events. This relationship does not hold in the HF treatment, indicating that the physical effects of increased DP/DO are superseded by other processes stimulating emissions of N2 and N2O. Previously collected pasture biomass data over multiple cuts demonstrated no response of biomass yield to different wetting and drying cycles (Mumford et al. 2019). With no differences in pasture growth, the relative increase in CO2 emissions in the HF treatment denotes an increase in microbial activity (Samad et al. 2016), inducing increased O2 consumption (Meyer et al. 2010; Rohe et al. 2021), which promotes N2 and N2O emissions via denitrifying pathways. This assumption is consistent with increased mineralisation in response to wetting and drying (Borken and Matzner 2009) and the subsequent increase in C and N and availability which promotes microbial activity. In the study presented here, neither DOC nor mineral N concentrations differed between treatments prior the simulated rainfall event. However, these concentrations only represent the balance between production and consumption and do not necessarily reflect actual substrate availability for microbial consumption. Our results suggest that the increase in microbial activity and related O2 consumption in response to small and repeated wetting events can offset the effects of increased DP/DO on denitrification, explaining the lack of treatment effect on cumulative N2O and N2 emissions. These findings also highlight the limitation of DP/DO as a sole predictor for N2O and N2 emissions and suggest that reductions of N2O emissions by increased irrigation frequency reported from cropping soils (Jamali et al. 2015) may not be transferrable to pasture soils characterised by large microbial biomass (Friedl et al. 2020b) and high organic C and N content.
Legacy effect of wetting and drying cycles on N2:N2O partitioning
The simulated rainfall event increased N2O emissions in both treatments, exhibiting high variability within treatments. However, the relative increase in N2O after rainfall was more pronounced in the LF treatment, also showing an increased shift of the N2O/(N2O + N2) towards N2O. Soil moisture in both treatments prior rainfall was comparable, meaning that antecedent soil moisture (Bergstermann et al. 2011) and thus the immediate effect of wetting did not cause the increased shift of the N2O/(N2O + N2) ratio towards N2O in the LF treatment. Long-term rainfall manipulations (Evans and Wallenstein 2012) and drought (Canarini et al. 2021) studies have shown that historical soil water content can alter the response of soil microbes to rainfall events. Previously observed reductions of N2O in the HF treatment during periods with rainfall only (Mumford et al. 2019) have been attributed to persistent legacy effects of wetting and drying cycles on ammonia oxidisers (Fierer and Schimel 2002), and NO3− substrate availability and its effect on the N2O/(N2O + N2) ratio (Friedl et al. 2020b; Senbayram et al. 2022). Gene copy numbers of archaeal and bacterial amoA did not show any treatment effects on the abundance of nitrifiers prior the experiment, nor did they respond to wetting and drying cycles during the experiment (Fig. 6). Even though the abundance of marker genes is not necessarily indicative for transcription, and further for enzyme activity, the data provide no indication that the postulated legacy effect is explained by the abundance of nitrifiers, or by differences in NO3− supply and/or NO3− availability. Nevertheless, future research should further investigate links between the structure of the N cycling microbial community, functional gene abundance and expression (Li et al. 2021), enzyme activity (Qin et al. 2017) and resulting N transformations in response to wetting and drying to expand the findings of the study presented here. Similar to nitrification, the abundance of nosZ carrying denitrifiers (clade I) showed no significant treatment response, and differences between treatments regarding the increase in N2O after the rainfall event were not reflected in nosZ abundance. It is noteworthy that the sole quantification of nosZ clade I provides only information on this particular clade, and the inclusion of recently developed primers covering a broader range of taxa for nosZ clade I (Zhang et al. 2021) and nosZ clade II (Hallin et al. 2018) may produce better correlations with N2O emissions (Xu et al. 2020) and their further reduction to N2. The abundance of nosZ carrying organisms as observed in the study presented here suggests that differences in N2O fluxes and the increased reduction to N2 are not explained by differences in abundance of nosZ carrying organisms, but by factors driving their response to large rainfall events. In contrast to N, DOC availability was higher in HF than LF after the rainfall event. This increase may be related to increased mineralisation prior the rainfall event as indicated by CO2 emitted from HF compared to LF (Fig. 4), with potential implications for N2O:N2 partitioning. Carbon is unlikely limiting for heterotrophic N2O producing and reducing organisms in this pasture soil with more than 2.5% organic C content. However, increased C availability has been linked to increased reduction of N2O to N2 (Friedl et al. 2021; Putz et al. 2018), which may explain the observed differences in N2O and the N2:N2O ratio, indicating an important link between C mineralisation (Dong et al. 2021) and the observed effect of historical wetting and drying cycles on N2O production and consumption in subtropical pasture soils.
Decoupling of N2O and N2 emissions
The temporal pattern of gas fluxes after the rainfall event shows a decoupling of N2O and N2 emissions, with N2 fluxes increasing to > 1000 g N2-N ha−1 day, and N2O emissions decreasing to values below 20 g N2O-N ha−1 day. Delayed emissions of N2 after rainfall events have been attributed to reduced DP/DO and subsequent entrapment of both N2O and N2 in the soil matrix (Clough et al. 2005). This is consistent with the high N2 fluxes observed in this study, implying the release of previously produced N2 as DP/DO increases after the rainfall event. This assumption is further supported by the low N2O emissions, as retention of N2O in the soil matrix favours complete denitrification to N2 (Hansen et al. 2014). Soil layers below 0.5 m depth in these heavy clay Vertisols under pasture rarely dry out when irrigated. Applied N and moisture can accumulate in these layers after rainfall, providing conditions favouring N2O and N2 production. The delayed peak of N2 fluxes observed in this study may therefore be attributed to the overlapping effects of gas entrapment and subsoil denitrification after rainfall, and future research should account for their effect on magnitude and temporal variability of N2 and N2O emissions from intensively managed pastures.
Conclusions
This study delivers critical data on how both N2O and N2 emissions respond to differences in wetting and drying cycles when water availability remains optimal for pasture growth. Repeated, small amounts of irrigation produced the same amount of N2 and N2O as a single and large irrigation event, highlighting that frequency, not only the amplitude of wetting and drying cycles drives the magnitude of N2 and N2O emissions from pasture soils. This study captured N2O and N2 emissions over a single grazing cycle, and further research is needed to expand our findings across a wider range of environmental conditions and to investigate the persistence of effects of historical wetting and drying regimes. Even though mechanisms are likely overlapping, we postulate that physical effects of reduced soil gas diffusivity (DP/DO) predominantly drive N2O and N2 emissions in response to single and large wetting events. Microbial activity and ensuing O2 consumption are however likely the main factors driving production of N2O and N2 in response to small but repeated wetting events. Our findings highlight the limitation of modelling approaches using only soil water content as a predictor for O2 availability when forecasting the response of N2O and N2 emissions to wetting and drying cycles. The lack of treatment effect on cumulative N2 and N2O emissions shows limited scope of increased irrigation frequency to reduce N2 and N2O triggered by irrigation from pasture soils characterised by high N turnover. However, the observed legacy effect of wetting and drying cycles after rainfall indicates that increased irrigation frequency can reduce N2O emissions and shift the N2O/(N2O + N2) ratio towards N2 following large rainfall events, reducing the environmental impact, but not the overall magnitude of N2O and N2 emissions from intensively managed pastures.
References
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56 FAO, Rome
Arah JRM (1997) Apportioning nitrous oxide fluxes between nitrification and denitrification using gas-phase mass spectrometry. Soil Biol Biochem 29:1295–1299. https://doi.org/10.1016/S0038-0717(97)00027-8
Azam F, Müller C, Weiske A, Benckiser G, Ottow J (2002) Nitrification and denitrification as sources of atmospheric nitrous oxide – role of oxidizable carbon and applied nitrogen. Biol Fertil Soils 35:54–61. https://doi.org/10.1007/s00374-001-0441-5
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial sourceas well as the magnitude of N2O emission from soil. Biol Fertil Soils 46:793–805. https://doi.org/10.1007/s00374-010-0484-6
Balaine N, Clough TJ, Beare MH, Thomas SM, Meenken ED (2016) Soil Gas Diffusivity Controls N2O and N2 Emissions and their Ratio Soil. Sci Soc Am J 80:529–540. https://doi.org/10.2136/sssaj2015.09.0350
Banerjee S, Helgason B, Wang L, Winsley T, Ferrari BC, Siciliano SD (2016) Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol Biochem 95:40–50. https://doi.org/10.1016/j.soilbio.2015.12.004
Bergstermann A, Cárdenas L, Bol R, Gilliam L, Goulding K, Meijide A, Scholefield D, Vallejo A, Well R (2011) Effect of antecedent soil moisture conditions on emissions and isotopologue distribution of N2O during denitrification. Soil Biol Biochem 43:240–250. https://doi.org/10.1016/j.soilbio.2010.10.003
Blagodatskaya E, Zheng X, Blagodatsky S, Wiegl R, Dannenmann M, Butterbach-Bahl K (2014) Oxygen and substrate availability interactively control the temperature sensitivity of CO2 and N2O emission from soil. Biol Fertil Soils 50:775–783. https://doi.org/10.1007/s00374-014-0899-6
Blagodatsky S, Smith P (2012) Soil physics meets soil biology: Towards better mechanistic prediction of greenhouse gas emissions from soil. Soil Biol Biochem 47:78–92. https://doi.org/10.1016/j.soilbio.2011.12.015
Borken W, Matzner E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob Change Biol 15:808–824
Braker G, Dörsch P, Bakken LR (2012) Genetic characterization of denitrifier communities with contrasting intrinsic functional traits. FEMS Microbiol Ecol 79:542–554. https://doi.org/10.1111/j.1574-6941.2011.01237.x
Canarini A, Schmidt H, Fuchslueger L, Martin V, Herbold CW, Zezula D, Gündler P, Hasibeder R, Jecmenica M, Bahn M, Richter A (2021) Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nat Commun 12:5308. https://doi.org/10.1038/s41467-021-25675-4
Clough T, Sherlock R, Rolston D (2005) A review of the movement and fate of N2O in the subsoil. Nutr Cycling Agroecosyst 72:3–11
Clough TJ, Cardenas LM, Friedl J, Wolf B (2020) Nitrous oxide emissions from ruminant urine: science and mitigation for intensively managed perennial pastures. Curr Opin Environ Sustain 47:21–27. https://doi.org/10.1016/j.cosust.2020.07.001
Čuhel J, Šimek M (2011) Proximal and distal control by pH of denitrification rate in a pasture soil. Agric Ecosyst Environ 141:230–233. https://doi.org/10.1016/j.agee.2011.02.016
De Rosa D, Rowlings DW, Fulkerson B, Scheer C, Friedl J, Labadz M, Grace PR (2020) Field-scale management and environmental drivers of N2O emissions from pasture-based dairy systems. Nutr Cycling Agroecosyst 117:299–315. https://doi.org/10.1007/s10705-020-10069-7
Dong H, Zhang S, Lin J, Zhu B (2021) Responses of soil microbial biomass carbon and dissolved organic carbon to drying-rewetting cycles: A meta-analysis. CATENA 207:105610. https://doi.org/10.1016/j.catena.2021.105610
Evans SE, Wallenstein MD (2012) Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 109:101–116. https://doi.org/10.1007/s10533-011-9638-3
Farquharson R, Baldock J (2008) Concepts in modelling N2O emissions from land use. Plant Soil 309:147–167
Fierer N, Schimel JP (2002) Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787. https://doi.org/10.1016/S0038-0717(02)00007-X
Friedl J, Scheer C, Rowlings DW, Mumford MT, Grace PR (2017) The nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) reduces N2 emissions from intensively managed pastures in subtropical Australia. Soil Biol Biochem 108:55–64. https://doi.org/10.1016/j.soilbio.2017.01.016
Friedl J, Cardenas LM, Clough TJ, Dannenmann M, Hu C, Scheer C (2020) Measuring denitrification and the N2O:(N2O+N2) emission ratio from terrestrial soils. Curr Opin Environ Sustain 47:61–71. https://doi.org/10.1016/j.cosust.2020a.08.006
Friedl J, Scheer C, Rowlings DW, Deltedesco E, Gorfer M, De Rosa D, Grace PR, Müller C, Keiblinger KM (2020) Effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on N-turnover, the N2O reductase-gene nosZ and N2O:N2 partitioning from agricultural soils. Sci Rep 10:2399. https://doi.org/10.1038/s41598-020-59249-z
Friedl J, Scheer C, De Rosa D, Müller C, Grace PR, Rowlings DW (2021) Sources of nitrous oxide from intensively managed pasture soils: the hole in the pipe. Environ Res Lett 16:065004. https://doi.org/10.1088/1748-9326/abfde7
Giles M, Morley N, Baggs EM, Daniell TJ (2012) Soil nitrate reducing processes - drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front microbiol 3:407–407. https://doi.org/10.3389/fmicb.2012.00407
Hallin S, Philippot L, Löffler FE, Sanford RA, Jones CM (2018) Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol 26:43–55. https://doi.org/10.1016/j.tim.2017.07.003
Hansen M, Clough TJ, Elberling B (2014) Flooding-induced N2O emission bursts controlled by pH and nitrate in agricultural soils. Soil Biol Biochem 69:17–24
Isbell R (2016) The Australian soil classification. CSIRO publishing, Collingwood, Victoria
Jamali H, Quayle WC, Baldock J (2015) Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling. Agric For Meteorol 208:32–39. https://doi.org/10.1016/j.agrformet.2015.04.010
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276. https://doi.org/10.1038/nrmicro.2018.9
Li B-B, Roley SS, Duncan DS, Guo J, Quensen JF, Yu H-Q, Tiedje JM (2021) Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biol Biochem 160:108349. https://doi.org/10.1016/j.soilbio.2021.108349
Meyer A, Bergmann J, Butterbach-Bahl K, Brüggemann N (2010) A new 15N tracer method to determine N turnover and denitrification of Pseudomonas stutzeri. Isot Environ Health Stud 46:409–421. https://doi.org/10.1080/10256016.2010.528840
Moldrup P, Deepagoda TKKC, Hamamoto S, Komatsu T, Kawamoto K, Rolston DE, de Jonge LW (2013) Structure-Dependent Water-Induced Linear Reduction Model for Predicting Gas Diffusivity and Tortuosity in Repacked and Intact Soil. Vadose Zone J 12:0026. https://doi.org/10.2136/vzj2013.01.0026
Morley N, Baggs EM, Dörsch P, Bakken L (2008) Production of NO, N2O and N2 by extracted soil bacteria, regulation by NO2− and O2 concentrations. FEMS Microbiol Ecol 65:102–112
Mumford MT, Rowlings DW, Scheer C, De Rosa D, Grace PR (2019) Effect of irrigation scheduling on nitrous oxide emissions in intensively managed pastures. Agric Ecosyst Environ 272:126–134. https://doi.org/10.1016/j.agee.2018.11.011
Petersen SO, Ambus P, Elsgaard L, Schjønning P, Olesen JE (2013) Long-term effects of cropping system on N2O emission potential. Soil Biol Biochem 57:706–712
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2019) nlme: linear and nonlinear mixed effects models. R package version 3.1-139.
Putz M, Schleusner P, Rütting T, Hallin S (2018) Relative abundance of denitrifying and DNRA bacteria and their activity determine nitrogen retention or loss in agricultural soil. Soil Biol Biochem 123:97–104. https://doi.org/10.1016/j.soilbio.2018.05.006
Qin S, Ding K, Clough TJ, Hu C, Luo J (2017) Temporal in situ dynamics of N2O reductase activity as affected by nitrogen fertilization and implications for the N2O/(N2O + N2) product ratio and N2O mitigation. Biol Fertil Soils 53:723–727. https://doi.org/10.1007/s00374-017-1232-y
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Rohe L, Apelt B, Vogel HJ, Well R, Wu GM, Schluter S (2021) Denitrification in soil as a function of oxygen availability at the microscale. Biogeosciences 18:1185–1201. https://doi.org/10.5194/bg-18-1185-2021
Rousset C, Clough TJ, Grace PR, Rowlings DW, Scheer C (2021) Irrigation Scheduling with Soil Gas Diffusivity as a Decision Tool to Mitigate N2O Emissions from a Urine-Affected Pasture. Agriculture 11:443
Samad MS, Bakken LR, Nadeem S, Clough TJ, de Klein CAM, Richards KG, Lanigan GJ, Morales SE (2016) High-Resolution Denitrification Kinetics in Pasture Soils Link N2O Emissions to pH, and Denitrification to C Mineralization. PLOS ONE 11:e0151713. https://doi.org/10.1371/journal.pone.0151713
Senbayram M, Wei Z, Wu D, Shan J, Yan X, Well R (2022) Inhibitory effect of high nitrate on N2O reduction is offset by long moist spells in heavily N loaded arable soils. Biol Fertil Soils 58:77–90. https://doi.org/10.1007/s00374-021-01612-x
Spott O, Russow R, Apelt B, Stange CF (2006) A 15N-aided artificial atmosphere gas flow technique for online determination of soil N2 release using the zeolite Köstrolith SX6®. Rapid Commun Mass Spectrom 20:3267–3274
Stepniewski W (1981) Oxygen diffusion and strength as related to soil compaction II. Oxygen diffusion coefficient. Polish J Soil Sci 14:3–13
Stres B, Danevčič T, Pal L, Fuka MM, Resman L, Leskovec S, Hacin J, Stopar D, Mahne I, Mandic-Mulec I (2008) Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol Ecol 66:110–122. https://doi.org/10.1111/j.1574-6941.2008.00555.x
IUSS WG (2015) World reference base for soil resources 2014 (update 2015). International Soil Classification System for Naming Soils and creating Legends for Soil Maps World Soil Resources Reports 106
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O (2018) The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3–A16. https://doi.org/10.1016/j.soilbio.2018.03.020
Xu X, Liu Y, Singh BP, Yang Q, Zhang Q, Wang H, Xia Z, Di H, Singh BK, Xu J, Li Y (2020) NosZ clade II rather than clade I determine in situ N2O emissions with different fertilizer types under simulated climate change and its legacy. Soil Biol Biochem 150:107974. https://doi.org/10.1016/j.soilbio.2020.107974
Zhang B, Penton CR, Yu Z, Xue C, Chen Q, Chen Z, Yan C, Zhang Q, Zhao M, Quensen JF, Tiedje JM (2021) A new primer set for Clade I nosZ that recovers genes from a broader range of taxa. Biol Fertil Soils 57:523–531. https://doi.org/10.1007/s00374-021-01544-6
Acknowledgements
We would like to thank Hao Long and Daniela Tikel for enabling and supporting work in the laboratories of QUT’s Centre for Agriculture and the Bioeconomy.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This study was supported by funding from the Australian Government Department of Agriculture and Water Resources as part of its Rural R&D for Profit programme and dairy Australia. The data reported in this paper were obtained at the Central Analytical Research Facility (CARF) at Queensland University of Technology (QUT). Access to CARF is supported by generous funding from the Science Faculty (QUT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Friedl, J., Deltedesco, E., Keiblinger, K.M. et al. Amplitude and frequency of wetting and drying cycles drive N2 and N2O emissions from a subtropical pasture. Biol Fertil Soils 58, 593–605 (2022). https://doi.org/10.1007/s00374-022-01646-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01646-9