Abstract
The effect of rapid rewetting of dry soil to a water content optimal for microbial activity on soil respiration and nutrient cycling has been extensively studied. However, with smaller rainfall events, dry soil may also rewet more slowly with an interval between partial rewetting events. In this experiment, soil unamended or amended with faba bean residue (C/N 9) was incubated at 50% water-holding capacity (WHC) for 2 weeks, then the soil of the rewet treatments was air-dried and incubated dry for 2 weeks. Then, the air-dried soil was exposed to six rewetting treatments: rewet rapidly to 50% WHC once on day 0 (RR50), rewet partially to 25% WHC once on day 0 (PR25), or rewet slowly by two applications of 25% WHC each with the first rewetting on day 0 and the second after 6 h (SR6), 12 h (SR12), 36 h (SR36), or 60 h (SR60). Constantly moist (CM) soil was kept at 50% WHC throughout the experiment. The flush of respiration after the final rewetting followed the order SR6 > RR50 > SR12 > SR36 > PR25, SR60. Microbial biomass C on the first day after the final rewetting decreased in the order RR50 > PR25 > SR6 > SR12 > SR 36 > SR60, CM. We conclude that slow rewetting can increase the flush of respiration compared to rapid rewetting, but only if the second partial rewetting occurs 6 h after the first. The size of the flush decreased with increasing time between the two rewetting events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long periods with little or no rain are characteristic for arid and semi-arid climate and impose stress on soil microbes through their effect on soil water availability. Soil drying reduces diffusion of substrate to microbes and water availability (Stark and Firestone 1995) because water potential becomes more negative; i.e. the remaining water is held more tightly on soil particles and water films around soil particles become thinner (Ilstedt et al. 2000). Low water availability has been shown to reduce microbial activity (Fierer and Schimel 2002) and change microbial community composition (Barnard et al. 2013; Fierer et al. 2003; Meisner et al. 2018). Microbes can survive in dry soil by becoming dormant or inactive, but they can also minimise cell desiccation by enhanced production of extra-cellular polysaccharides as a protective coating (Kakumanu et al. 2013) or by accumulation of solutes to retain turgor (Or et al. 2007; Warren 2014).
Dry periods may be interrupted or ended by rainfall events that also is a stress for microbes if dry soil is rapidly rewet. Rapid rewetting of air-dried soil to a water content optimal for microbial activity (in most soils about 50% of maximum water holding capacity) results in a flush of CO2 and available nutrients, which is referred to as “Birch effect” (Barnard et al. 2020; Birch 1958) and is due to increased substrate availability to surviving microbes. Biotic substrate sources include microbes killed in dry soil or upon rewetting, release of intracellular osmoregulatory compounds accumulated during the dry period (Blazewicz et al. 2014, 2020), aggregate breakdown which exposes organic matter previously inaccessible to soil microorganisms (Hueso et al. 2012; Najera et al. 2020; Wu and Brookes 2005) and water-extractable OC accumulated in dry soils (Kaiser et al. 2015) which can be released upon rewetting of dry soil (Slessarev et al. 2020). Abiotic CO2 sources include the release of CO2 accumulated in dry soil as water enters soil pores and solubilisation of carbonates (Barnard et al. 2020). Exposure to several dry-rewet (DRW) cycles has been shown to increase microbial resilience to DRW as expressed in a rapid increase in respiration upon rewetting and high C use efficiency, i.e. low respiration/growth ratio (Brangari et al. 2021; de Nijs et al. 2019). This has been explained by metabolic adaptation or changes in microbial community composition (Brangari et al. 2021; Koch et al. 2018). Due to the large extent of arid and semi-arid areas and thus their impact on the global C cycle (Ahlstrom et al. 2015), it is important to understand the impact of dry periods and rewetting events on CO2 release from soil.
Rainfall events interrupting or ending dry periods vary in intensity, but only few studies have examined the effect of less dramatic changes in soil water content than those described above. For example, Chowdhury et al. (2011) reported that the flush of respiration after rewetting to optimal water content decreased with increasing previous water content. Studying the effect of different rainfall amounts (1, 5 or 15 mm) in the middle of a dry season, Rey et al. (2017) found that CO2 emission was positively related to the amount of rainfall. These studies have exposed soils to single water addition. But dry soils could also be rewet gradually by several small rainfall events. In a previous study, we exposed dry soil to three rewet treatments: rapid rewetting to 50% maximum water-holding capacity (WHC), partial rewetting to 25% WHC and slow rewetting where water was added in two aliquots corresponding to 25% WHC each with 6 h between the first and the second rewetting (Erinle et al. 2021). The flush of respiration after the final rewetting and cumulative respiration were greatest with slow rewetting and were 30% higher than with rapid rewetting. We explained the high respiration with slow rewetting by activation of the soil microbes by the first rewetting event which allows them to respond quickly to substrates becoming available with the second rewetting. However, small rainfall events may be separated by more than 6 h and the length of the interval between small rewetting events could influence the capacity of the soil microbes to respond to the second rewetting.
The aim of this study was to determine the effect of different intervals between two rewetting events (6 h, 12 h, 36 h, 60 h, referred to as slow rewetting) on soil respiration and N availability after a second rewetting event in soil that was unamended or amended with low C/N faba bean residue. The amendment was added to increase substrate availability to microbes. The following hypotheses were tested: (1) compared to rapid rewetting, the flush of respiration after slow rewetting will be greater if the second rewetting event occurred within a day after the first; (2) among the slow rewetted treatments, the longer the rewetting interval, the lower the respiration rate and microbial biomass C upon the second rewetting event, because microbial activity that was increased by the first partial rewetting was limited by low substrate and water availability between the first and the second rewetting event; and (3) differences among treatments will decrease over time after the second rewetting event. The first hypothesis is based on our previous study (Erinle et al. 2021) which showed that the flush of respiration was greater when second rewetting event occurred 6 h after the first than with a single rewetting with the same total volume of water added. The third hypothesis assumes that substrate will be depleted during the experiment and therefore limit microbial activity towards the end of the experiment.
Materials and methods
Soil and crop residue
The properties of the soil used in this experiment are described in Erinle and Marschner (2019). The loamy sand (0 to 10 cm depth) was collected from Waite Campus of The University of Adelaide, South Australia (longitude 138° 38′ E, latitude 35° 6′ S); the site had been under permanent pasture over 80 years but recently cropped with oats. This area has a Mediterranean climate: cool, wet winters and hot, dry summers with occasional short, heavy rainfall events. The soil is classified as Chromosol in Australian soil classification (Isbell 2002) and Lixisol in WRB (FAO 2015). Soil (0–10 cm) collected from six different randomly selected sites was combined and mixed. The soil was dried at 40 °C and sieved to < 2 mm. The soil properties are pH 6.8 (1:5 soil/water); EC (1:5) 0.1 dS m−1; clay 25%, silt 37%, sand 37%; total P 302 mg kg−1; total organic C 17 g kg−1; total organic N 1.5 g kg−1; bulk density 1.3 g cm−3; and maximum water holding capacity (WHC) 350 g kg−1.
Young faba bean shoot (Vicia faba L.), dried at 40 °C and ground and sieved to 0.25–2-mm particle size, was used as amendment. It has the following properties: total organic C, 347 g kg−1; total N, 38.5 g kg−1; and C/N ratio 9 (Erinle and Marschner 2019).
Twenty-gramme dry soil left unamended or amended with faba bean (10 g kg−1) was placed into small plastic bags and mixed thoroughly with 175 g reverse osmosis (RO) water kg−1 (50% WHC). Then, the mixed soil was filled into small PVC cores (1.8 cm radius, 5 cm high) with a mesh bottom and adjusted to a bulk density of bulk density 1.3 g cm−3.
Experimental design
Soil was maintained at 50% WHC and incubated in the dark at room temperature for 2 weeks. Thereafter, individual cores were placed in 1-L glass jars with gas tight lid that had a septum to allow gas sampling. Then, the soil of the drying and rewetting treatments was dried to < 5% WHC using silica pouches (Butterly et al. 2009) and incubated for another 2 weeks. After 2 weeks, the dry soils were rewet rapidly to 50% WHC once on day 0 (RR50), rewet partially to 25% WHC on day 0 (PR25) or rewet slowly by two applications to 25% WHC each with the first rewetting on day 0 and the second after 6 h (SR6), 12 h (SR12), 36 h (SR36) or 60 h (SR60) (Table 1). The soils were incubated moist for 2 weeks after the initial rewetting. Constantly, moist (CM) soil was kept at 50% WHC throughout the experiment. Soil respiration was measured daily from 1 day before rewetting to day three after rewetting, and subsequently measured every 2 days, because soil respiration rate changed little over 24 h. Available N, microbial biomass C (MBC) and K2SO4-extractable NH4 as indicator of microbial biomass N were measured 1, 2, 3, 7 and 14 days after the initial rewetting. In the first 3 days, CM, RR50 and PR25 were sampled on day 1, 2, and 3, but treatments that were rewet twice were only sampled the day after the second rewetting. Thus, data for rewet treatments of the first 3 days are from day 1 for RR50, PR25, SR6 and SR12, day 2 for SR36 and day 3 for SR60. For CM, the average of days 1–3 is used. Daily data for CM, RR50 and PR25 in the first 3 days is shown in the supplementary table (Table S1).
Analyses
Measurements were carried out as described in Erinle and Marschner (2019). Soil maximum water holding capacity was measured, matric potential = − 10 kPa (Wilke 2005). Soil texture was determined according to Gee and Or (2002). Soil pH was determined in a 1:5 (w/v) soil to reverse osmosis (RO) water ratio (Rayment and Higginson 1992). Total organic C of soil and residues was determined by wet oxidation (Walkley and Black 1934). Total N in soil and plant residues was determined using the Kjeldahl method (Bremner and Mulvaney 1982). Soil respiration was measured by quantifying the CO2 concentration in the headspace of the jars using a Servomex 1450 infra-red analyser (Servomex Group, Crowborough, UK) as described in Setia et al. (2011).
Available N (exchangeable ammonium and nitrate) was measured after 1 h end-over-end shaking with 2 M KCl in a 1:5 soil-extractant ratio. Ammonium-N was determined after Willis et al. (1996) and nitrate–N after Miranda et al. (2001). Microbial biomass N and C (MBN and MBC) were determined by chloroform fumigation extraction with 0.5 M K2SO4 (Moore et al. 2000; Vance et al. 1987). Chloroform-labile C concentration is the difference in organic C between fumigated and non-fumigated soil, which was multiplied by 2.64 to calculate MBC (Vance et al. 1987). The respiration rate/MBC ratio was calculated as a measure of C use efficiency. K2SO4-extractable organic C of the unfumigated soil was used as indicator of extractable C. The difference in concentration of K2SO4-extractable NH4+ between fumigated and non-fumigated samples was used as indicator for microbial biomass N without using the conversion factor suggested by Moore et al. (2000).
Statistical analysis
For available N, MBC and K2SO4-extractable NH4, average values over first 3 days were used for CM. For all rewetted treatments, data of 1 day after rewetting is shown. For treatments that were rewet twice, we used data of the first day after the final rewetting (day 1 for RR50, PR25, SR6 and SR12, day 2 for SR36, day 3 for SR60). Data of weekly cumulative respiration, available N, MBN and MBC was checked for normality using the Shapiro–Wilk test. After confirming normality, the data was analysed by two-way repeated measures ANOVA with fixed factors amendment (unamended and faba bean residue) and moisture treatment (constantly moist, rapid rewetting to 50% WHC, partial rewetting to 25% WHC, slow rewetting to 50% WHC with 6 h, 12 h, 36 h or 60 h interval between the two rewetting events), and time as repeated measure. Tukey’s multiple comparison test was used to determine significant differences for the amendment × moisture treatment interaction (P ≤ 0.05). The statistical analyses were carried out in Genstat v19 (VSN International Ltd., UK).
Results
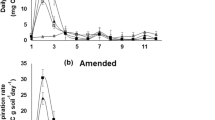
The respiration rate in the first 5 days was generally higher in amended than unamended treatments and differences among treatments were greater in amended treatments (Fig. 1a, b). On day 1, the respiration rate was highest in SR6 and lowest in CM. Compared to CM, the respiration rate was fivefold higher in SR6, fourfold higher in RR50, threefold higher in SR12 and about twofold higher in SR36, SR60 and PR25. The respiration rate decreased in all rewet treatments from day 1 to day 2 except in SR36 where it remained unchanged. On day 2 compared to CM, the respiration rate was threefold higher in RR50, SR6, SR12 and SR36, twofold higher in SR60 but it did not differ between CM and PR25. From day 2 to day 3, the respiration rate changed little in most treatments, except in RR50 where it decreased by about 30% and in SR36 in amended soil, where it increased by about 25%. On day 3, the respiration rate was highest in SR36, it was about twofold higher than in RR50, CM and PR25 and about 25% higher than in SR6, SR12 and SR50. Respiration rates in all treatments decreased further until day 5 after which they remained stable until the end of the experiment.
Respiration rates (A–B) and cumulative respiration (C) from 0 to 14 days after rewetting in soils incubated dry for 14 days before rewetting rapidly to 50% WHC only once (RR50), partially to 25% WHC only once (PR25), or slowly to 50% WHC by two applications at 25% WHC each with 6 h (SR6), 12 h (SR12), 36 h (SR36) or 60 h (SR60) interval between the two rewetting events, or maintained constantly moist (CM), without amendment (unamended) or with faba bean residue. In panel C, for each period (0–3, 4–7 and 8–14 days) separately, lower-case letters indicate significant differences for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). Upper-case letters indicate significant differences in total cumulative respiration for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3)
Cumulative respiration in all moisture treatments was higher in amended than unamended soils (Fig. 1c). Cumulative respiration in the first 3 days was lowest in CM. Among rewetted treatments, it was lowest in PR25. In the unamended treatments, cumulative respiration in the first 3 days was higher in RR50 than the treatments that were rewet slowly. But in amended soil, it was about 20% higher in SR6 than RR50 and it decreased with increasing time between the two rewetting events. Cumulative respiration between day 4 and day 7 differed little among treatments in unamended soil, but in amended soils, it was higher in all treatments that were rewet twice compared to RR50, particularly in SR60 where it was twofold higher than RR50. Cumulative respiration from day 8 to day 14 differed little among moisture treatments. In amended soils compared to CM and PR25, cumulative respiration in the first 7 days was about 50% higher in RR50 and all treatments that were rewet twice. From day 8 to day 14, cumulative respiration was lowest in PR25 and differed little among the other treatments. Total cumulative respiration (days 0–14) in unamended soils was higher than CM only in RR50. In amended soils, it was about 75% higher in RR50 and all treatments that were rewet twice than CM and PR25.
In the first 3 days, MBC was higher in amended than unamended soils, about 25% higher in CM, RR50, PR25, 0.5-fold higher in SR6 and SR12 and twofold higher in SR36 and SR60 (Fig. 2a, Table S1). In unamended soil, MBC was lowest in SR60 where it was 75% lower than CM. Compared to CM, MBC in the first 3 days was about twofold higher in RR50, PR25 and SR6, 0.75-fold higher in SR12 and similar in SR36. In amended soil, MBC differed little between CM and SR60, but it was about threefold higher in RR50, PR25 and SR6, twofold higher in SR12 and 0.5-fold higher in SR36. MBC on day 7 was slightly higher in amended than unamended soil, but differed little among moisture treatments (Fig. 2b). It was generally lower than on days 1–3 except in SR36 and SR60 in unamended soil where it was 0.1- and fourfold higher, respectively. MBC increased up to twofold from day 7 to day 14. On day 14, MBC in unamended soil was lower in PR25 than the other treatments which differed little in MBC (Fig. 2c). In amended soil, MBC was lowest in CM and 0.3- to twofold higher in the rewet treatments.
Microbial biomass C (mg kg soil−1) on days 1, 2, 3, 7 and 14 after rewetting of soil previously dry for 14 days before rewetting rapidly to 50% WHC only once (RR50), partially to 25% WHC only once (PR25) or slowly to 50% WHC by two applications at 25% WHC each with 6 h (SR6), 12 h (SR12), 36 h (SR36) or 60 h (SR60) interval between the two rewetting events, or maintained constantly moist (CM), without amendment (unamended) or amended with faba bean residue. For each period separately, columns with different lower-case letters indicate significant differences for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). Upper-case letters indicate significant differences in total cumulative respiration for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). The * indicates an increase and # indicates a decrease in concentration compared to previous sampling time. Data of CM, RR50 and PR25 in panel A is average of days 1, 2 and 3
The respiration rate/MBC ratio in the first 3 days was about 1.5-fold higher in SR6 and SR36 than in the other treatments (Table 2). Compared to SR6, the respiration rate/MBC ratio in SR60 was twofold higher in unamended soil, but similar in amended soil. Among rewet treatments in unamended soil, it was lowest in PR25. On day 7, the respiration rate/MBC ratio was lowest in SR6 and highest in RR50 while it did not differ in the other treatments. The respiration rate/MBC ratio on day 14 was lower in SR6 and SR12 than the other rewet treatments.
Extractable organic C (OC) (determined as K2SO4-extractable OC from unfumigated soil, Supplementary Fig. 1) differed little between amended and unamended soils except for CM in the first 3 days where extractable OC was about sixfold higher in amended than unamended soil (Fig. S1a). Among rewetted treatments, extractable OC was higher in SR36 and SR60 than in RR50 and PR25, particularly in unamended soil. Treatments differed little in extractable OC on days 7 and 14 (Fig. S1b,c).
In the first 3 days, K2SO4-extractable NH4 as indicator of microbial biomass N was about twofold higher in amended than unamended soil in most rewet treatments except in SR60 where it did not differ between amended and unamended soil (Fig. 3a, Table S1). In both amendment treatments, K2SO4-extractable NH4 was lowest in SR60 followed by CM where it was about threefold higher. Compared to CM, K2SO4-extractable NH4 in unamended soil was about 25% higher in RR50 and PR25, similar in SR6, 30% lower in SR12, and about 80% lower in SR36 and SR60. In amended soil compared to CM, K2SO4-extractable NH4 in the first 3 days was fourfold higher in RR50 and PR25 and twofold higher in SR6 and SR12, similar in SR36 and about 50% lower in SR60. In unamended soil, K2SO4-extractable NH4 decreased from days 1–3 to day 7 in most treatments except in SR36 and SR60 where it increased twofold. In amended soil, K2SO4-extractable NH4 increased about twofold from days 1–3 to day 7 in CM and SR60, but decreased by about 20% in RR50, PR25, SR6 and SR12. In unamended soil, K2SO4-extractable NH4 on day 7 was up to 50% higher in CM than in the rewet treatments (Fig. 3b). In amended soil, K2SO4-extractable NH4 on day 7 was 2- and 1.5-fold higher in RR50 and PR25 than the other treatments, respectively. In unamended soil, K2SO4-extractable NH4 remained unchanged from day 7 to day 14, but it generally decreased in amended soil with the greatest decrease in RR50 and PR25 where it decreased threefold. Treatments differed little in K2SO4-extractable NH4 on day 14 (Fig. 3c).
K2SO4-extractable ammonium (mg kg soil−1) on days 1–3, 7 and 14 after rewetting of soil previously dry for 14 days before rewetting rapidly to 50% WHC only once (RR50), partially to 25% WHC only once (PR25) or slowly to 50% WHC by two applications at 25% WHC each with 6 h (SR6), 12 h (SR12), 36 h (SR36) or 60 h (SR60) interval between the two rewetting events, or maintained constantly moist (CM), without amendment (unamended) or amended with faba bean residue. For each period separately, columns with different lower-case letters indicate significant differences for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). Upper-case letters indicate significant differences in total cumulative respiration for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). The * indicates an increase and # indicates a decrease in concentration compared to previous sampling time. Data of CM, RR50 and PR25 in panel A is average of days 1, 2 and 3
Throughout the experiment, available N was higher in amended than unamended soil, about 50% higher on days 1–3 and up to twofold higher on days 7 and 14 (Fig. 4, Table S1). Available N in unamended soil in the first 3 days was about 20% higher in rewet treatments than in CM (Fig. 4a). In amended soil, available N was lowest in SR6 and about 20% higher in the other treatments. Available N on day 7 was about 50% lower than in the first 3 days (Fig. 4b). Available N changed little from day 7 to day 14 (Fig. 4c). On days 7 and 14, available N was lowest in CM; differences among rewet treatments were small.
Available N (mg kg soil−1) on days 1–3, 7 and 14 after rewetting of soil previously dry for 14 days before rewetting rapidly to 50% WHC only once (RR50), partially to 25% WHC only once (PR25) or slowly to 50% WHC by two applications at 25% WHC each with 6 h (SR6), 12 h (SR12), 36 h (SR36) or 60 h (SR60) interval between the two rewetting events, or maintained constantly moist (CM), without amendment (unamended) or amended with faba bean residue. For each period separately, columns with different lower-case letters indicate significant differences for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). Upper-case letters indicate significant differences in total cumulative respiration for the amendment × moisture treatment interaction (P ≤ 0.05, n = 3 ± standard error). The * indicates an increase and # indicates a decrease in concentration compared to previous sampling time. Data of CM, RR50 and PR25 in panel A is average of day 1, 2 and 3
Discussion
This study showed that the time between two partial rewetting events influenced respiration, MBC and MBN, with a more pronounced effect in amended than unamended soil. The greater effect in amended soil can be explained by the higher substrate availability where little decomposition occurred in the two dry weeks prior to the first rewetting. In unamended soil, low substrate availability likely limited microbial response to rewetting.
The first hypothesis (compared to rapid rewetting, the flush of respiration after slow rewetting will be greater if the second rewetting event occurred within a day after the first) has to be declined because the respiration flush was higher than RR50 only in SR6 whereas it was lower in SR12.
The second hypothesis (among the slow rewetted treatments, the longer the rewetting interval, the lower soil respiration and MBC) can be confirmed which will be discussed in detail below. The third hypothesis (differences among treatments will decrease over time after the second rewetting event) can also be confirmed. The pronounced differences in respiration and MBC among rewetting treatments in the first 3 days became smaller or disappeared on days 7 and 14.
With rapid rewetting to 50% WHC (RR50), the soil throughout the container is rewet quickly leading to osmotic stress, release of substrates for surviving microbes and a flush of respiration (Barnard et al. 2020; Birch 1958). Sources of the increased substrate availability include microbes killed in dry soil or during rewetting, aggregate breakdown and release of organic matter bound to soil particles (Barnard et al. 2020; Blazewicz et al. 2014; Patel et al. 2021). The flush of respiration 1 day after rewetting indicates a rapid activation of soil microbes corresponding to a type 1 response (Meisner et al. 2015). Rewetting also induced a strong increase in MBC compared to CM. This increase in respiration and microbial growth after rewetting of dry soil to optimal water content is consistent with previous studies (e.g. Meisner et al. (2015)). The smaller increase in K2SO4-extractable NH4 after rewetting compared to the increase in MBC in unamended soil suggests that microbes were N limited. In contrast, both MBC and K2SO4-extractable NH4 in amended soil increased to a similar extent compared to CM, indicating that sufficient N was available for microbes which is corroborated by the higher N availability in amended than unamended soil. In agreement with previous studies, respiration rates decreased rapidly after the initial flush (Barnard et al. 2020; Fierer and Schimel 2003).
With partial rewetting to 25% WHC (PR25), the lower amount of water added may have briefly increased water availability to optimal for microbes in the top few millimetres of the soil. But as the water moved down, water availability and hence osmotic stress for microbes would have been lower than in RR50 because the water potential difference to air dried soil was smaller due to the lower amount of water added in R25. This smaller stress may explain why the increase in MBC and K2SO4-extractable NH4 on day 1 was similar in PR25 and RR50. Hence, although the flush of respiration was smaller in PR25, C use efficiency was greater as indicated by the lower respiration rate/MBC ratio on day 1. However, the lower MBC and K2SO4-extractable NH4 on days 7 and 14 in PR25 compared to RR50 indicates that the lower soil moisture limited access of microbes to substrate.
In treatments with slow rewetting, water availability shortly after the first partial rewetting activated a proportion of the microbiome but also limited subsequent substrate availability. When the second rewetting occurred 6 h after the first (SR6), microbes activated by the first rewetting were likely still active and thus able to immediately utilise substrate that became available after the second rewetting as water penetrated more pores and the water film around the aggregates became thicker. This can explain the high respiration rate on day 1 (measured 18 h after second rewetting) which was twofold higher than PR25 corresponding to the water content after the first rewetting and about 20% higher than RR50 which had the same water content. The increase in MBC on day 1 in SR6 compared to CM was only slightly smaller than in RR50. However, C use efficiency was lower in SR6 than RR50 as indicated by the higher respiration rate/MBC ratio, possibly because less time had passed since the last rewetting (18 h compared to 24 h), and hence, respiration rates were still higher. But in amended soil, the increases in K2SO4-extractable NH4 and available N on days 1 and 7 compared to CM were smaller in SR6 than in RR50. This indicates that microbes in SR6 had less access to organic N possibly because there was less aggregate breakdown and release of bound organic matter than in RR50 (Najera et al. 2020). The smaller increase in K2SO4-extractable NH4 than in RR50 is in contrast to our previous study (Erinle et al. 2021) with soil collected from the same site where K2SO4-extractable NH4 was higher in the treatment corresponding to SR6 than in the treatment corresponding to RR50 whereas available N was lower. However, K2SO4-extractable NH4 and available N were lower in the previous study than in the current study suggesting that microbes were more N limited in the former. The soil was stored dry for a year between the two experiments. Although microbial activity is low in dry soil, N mineralisation may have occurred by microbes tolerant to low water content (Parker and Schimel 2011; Schaeffer et al. 2017), as well as by extracellular enzymes located in small, still water-filled pores (Blankinship et al. 2014). Due to the higher N availability in the present study, up to fourfold differences in K2SO4-extractable NH4 among treatments 1 day after the final rewetting had no impact on available N which differed little among treatments.
We had hypothesised that as time between rewetting events increases, microbial activity that was increased after the first rewetting would become low due to limited water and substrate availability. Additionally, substrate availability may be low because of decomposition after the first rewetting. Then, after the second rewetting event, microbes are activated again, but respiration would be lower than with 6 h between events (hypothesis 2). This hypothesis was supported by the lower respiration rate and lower MBC on day 1 in SR12 (12 h between the two rewetting events) compared to SR6. However, the ratio of respiration rate/MBC was lower in SR12 than SR6. Thus, with the longer interval between rewetting events, the microbes utilised C more efficiently after the second rewetting. A possible explanation is that the 12 h at 25% WHC allowed activation of microbial genotypes that utilised C more efficiently whereas the 6-h interval in SR6 was too short for this to occur. Previous studies showed that microbial community composition changes within a day after rewetting (Blazewicz et al. 2020; Liu et al. 2019). The greater C utilisation efficiency in SR12 may also be due to activation of previously inactive bacteria by rewetting (Engelhardt et al. 2019). The longer interval between the two rewetting events had little effect on N mineralisation and immobilisation as available N and K2SO4-extractable NH4 differed little between SR6 and SR12.
With 36 h between the two rewetting events (SR36), the flush of respiration was similar as in SR12 in amended soils, but smaller in unamended soils. This suggests that in amended soil despite the low water content, the soil microbes were able to maintain a relatively high activity over 36 h after the first partial rewetting due to the increased substrate availability. Thus, they could rapidly respond to additional substrate released by the second rewetting. In unamended soil on the other hand, the low water content apparently limited microbial activity to such an extent that they could not fully take advantage of the increased substrate availability after the second rewetting. Although the flush of respiration in SR36 in amended soil was similar as in SR12, MBC and K2SO4-extractable NH4 on the first day after the second rewetting were lower than in SR12 in both amended and unamended soil. Thus, C use efficiency was lower in SR36; i.e. the respiration rate/MBC ratio was higher than in SR12.
The smaller respiration flush and low MBC and K2SO4-extractable NH4 in SR60 (60 h between the two rewetting events) than SR12 can be explained by the low activity of the microbes after day 2 as indicated by the low respiration rate in PR25. Additionally, the higher respiration rate after the first rewetting than in air-dry soil (corresponding to that measured on day 0 before rewetting) indicates that some decomposition occurred between the two rewetting events which would reduce substrate availability after the second rewetting, particularly with the 60-h interval between the two rewetting events and in unamended soil. The ratio of respiration rate/MBC was higher in SR60 than SR12 due to the very low MBC in SR60, particularly in unamended soil. This suggests that microbial growth after the second rewetting in SR60 was strongly C limited while the energy generated by respiration was used for survival mechanisms and cellular repair (Tiemann and Billings 2011).
Compared to the pronounced differences in MBC and respiration in the first 3 days among rewetting treatments, differences in extractable OC were small. Thus, substrate availability did not seem to limit microbial growth or respiration. However, extractable OC does not reflect substrate availability to microbes in rewetted treatments in the first 3 days because the extraction is carried out at a 1:4 soil:extractant ratio. In the soil between the two rewetting events, microbial substrate access was limited due to the low water content leading to accumulation of water-extractable OC (Kaiser et al. 2015). The accumulated OC released upon rewetting soil (Slessarev et al. 2020) was then utilised for respiration and growth. This strong fluctuation in OC in rewetted treatments is not reflected in extractable OC.
Although the size of the respiration flush after the second rewetting and microbial biomass decreased with increasing time between the two rewetting events, total cumulative respiration during the 2 weeks differed little among slow rewetting treatments. Thus, with longer interval, the lower cumulative respiration in the first 7 days was mostly compensated by higher cumulative respiration between day 7 and day 14.
This experiment was conducted without growing plants. Presence of plants is likely to affect the influence of rewetting of dry soil due to root exudates which serve as substrate for microbes and competition between plants and microbes for nutrients and water which in turn are influenced by soil water availability (Dijkstra et al. 2012).
Conclusion
Slow rewetting can increase the flush of respiration compared to rapid rewetting, but not microbial biomass. However, the respiration flush was only higher than with rapid rewetting if the second partial rewetting event occurred 6 h after the first. A longer interval resulted in a lower respiration flush and lower microbial biomass. Nevertheless, total cumulative respiration differed little among slow rewetting treatments. Together with the similar MBC among moisture treatments on days 7 and 14, this indicates that a longer interval between partial rewetting events is unlikely to strongly influence soil respiration and microbial biomass in the longer term compared to rapid rewetting. In this study, the soil was exposed to only one dry-rewet cycle. With rapid rewetting of dry soil, repeated exposure to drying and rewetting events has been shown to reduce the size of the respiration flush which has been explained by substrate depletion and adaptation of soil microbes (Evans and Wallenstein 2012; Fierer et al. 2003; Mikha et al. 2005). Future studies could investigate if the effect of repeated slow rewetting events follows the same pattern. Also of interest would be to assess which microbial genotypes are active, dormant or dead during the dry period and after rewetting.
References
Ahlstrom A, Raupach MR, Schurgers G, Smith B, Arneth A, Jung M, Reichstein M, Canadell JG, Friedlingstein P, Jain AK, Kato E, Poulter B, Sitch S, Stocker BD, Viovy N, Wang YP, Wiltshire A, Zaehle S, Zeng N (2015) The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 348:895–899. https://doi.org/10.1126/science.aaa1668
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. Isme J 7:2229. https://doi.org/10.1038/ismej.2013.104
Barnard RL, Blazewicz SJ, Firestone MK (2020) Rewetting of soil: revisiting the origin of soil CO2 emissions. Soil Biol Biochem 147. https://doi.org/10.1016/j.soilbio.2020.107819
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10:9–31. https://doi.org/10.1007/bf01343734
Blankinship JC, Becerra CA, Schaeffer SM, Schimel JP (2014) Separating cellular metabolism from exoenzyme activity in soil organic matter decomposition. Soil Biol Biochem 71:68–75. https://doi.org/10.1016/j.soilbio.2014.01.010
Blazewicz SJ, Schwartz E, Firestone MK (2014) Growth and death of bacteria and fungi underlie rainfall-induced carbon dioxide pulses from seasonally dried soil. Ecology 95:1162–1172. https://doi.org/10.1890/13-1031.1
Blazewicz SJ, Hungate BA, Koch BJ, Nuccio EE, Morrissey E, Brodie EL, Schwartz E, Pett-Ridge J, Firestone MK (2020) Taxon-specific microbial growth and mortality patterns reveal distinct temporal population responses to rewetting in a California grassland soil. Isme J 14:1520–1532. https://doi.org/10.1038/s41396-020-0617-3
Brangari AC, Manzoni S, Rousk J (2021) The mechanisms underpinning microbial resilience to drying and rewetting - a model analysis. Soil Biol Biochem 162:108400
Bremner JM, Mulvaney C (1982) Nitrogen - total, methods of soil analysis. Part 2. . In: JM Bremner, C Mulvaney (Eds) Chemical and microbiological properties. American Society of Agronomy, Madison WI, pp 595–24
Butterly CR, Buenemann EK, Mcneill A, Baldock JA, Marschner P (2009) Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem 41:1409–1416
Chowdhury N, Burns RG, Marschner P (2011) Recovery of soil respiration after drying. Plant Soil 348:269–279. https://doi.org/10.1007/s11104-011-0871-2
de Nijs EA, Hicks LC, Leizeaga A, Tietema A, Rousk J (2019) Soil microbial moisture dependences and responses to drying-rewetting: the legacy of 18 years drought. Glob Chang Biol 25:1005–1015. https://doi.org/10.1111/gcb.14508
Dijkstra FA, Augustine DJ, Brewer P, von Fischer JC (2012) Nitrogen cycling and water pulses in semiarid grasslands: are microbial and plant processes temporally asynchronous? Oecologia 170:799–808. https://doi.org/10.1007/s00442-012-2336-6
Engelhardt IC, Blazewicz SJ, Barnard RL (2019) Effects of contrasting precipitation patterns on the trajectory of actively growing and inactive microbial communities after rewetting. Soil Biol Biochem 134:172–174. https://doi.org/10.1016/j.soilbio.2019.03.029
Erinle KO, Marschner P (2019) Soil water availability influences P pools in the detritusphere of crop residues with different C/P ratios. J Soil Sci Plant Nutr 19:771–779. https://doi.org/10.1007/s42729-019-00076-1
Erinle KO, Bengtson P, Marschner P (2021) Rewetting intensity influences soil respiration and nitrogen availability. Journal Soil Sci Plant Nutr 21:2137–2144. https://doi.org/10.1007/s42729-021-00509-w
Evans SE, Wallenstein MD (2012) Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 109:101–116. https://doi.org/10.1007/s10533-011-9638-3
FAO (2015) World reference base for soil resources. RAO, Rome
Fierer N, Schimel JP (2002) Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787. https://doi.org/10.1016/s0038-0717(02)00007-x
Fierer N, Schimel JP (2003) A proposed mechanism for the pulse in carbon dioxide production commonly abserved following the rapid rewetting of a dry soil. Soil Sci Soc Amer J 67:798–805
Fierer N, Schimel JP, Holden PA (2003) Influence of drying–rewetting frequency on soil bacterial community structure. Microb Ecol 45:63–71. https://doi.org/10.1007/s00248-002-1007-2
Gee GW, Or D (2002) Particle-size analysis. In: JH Dane, CG Topp (Eds) Methods of soil analysis Part 4 Physical methods. Soil Science Society of America, Madison, WI, pp 255–293
Hueso S, García C, Hernández T (2012) Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol Biochem 50:167–173. https://doi.org/10.1016/j.soilbio.2012.03.026
Ilstedt U, Nordgren A, Malmer A (2000) Optimum soil water for soil respiration before and after amendment with glucose in humid tropical acrisols and a boreal mor layer. Soil Biol Biochem 32:1594–1599
Isbell R (2002) The Australian Soil Classification. CSIRO Publishing
Kaiser M, Kleber M, Berhe AA (2015) How air-drying and rewetting modify soil organic matter characteristics: an assessment to improve data interpretation and inference. Soil Biol Biochem 80:324–340. https://doi.org/10.1016/j.soilbio.2014.10.018
Kakumanu ML, Cantrell CL, Williams MA (2013) Microbial community response to varying magnitudes of desiccation in soil: a test of the osmolyte accumulation hypothesis. Soil Biol Biochem 57:644–653. https://doi.org/10.1016/j.soilbio.2012.08.014
Koch BJ, McHugh TA, Hayer M, Schwartz E, Blazewicz SJ, Dijkstra P, van Gestel N, Marks JC, Mau RL, Morrissey EM, Pett-Ridge J, Hungate BA (2018) Estimating taxon-specific population dynamics in diverse microbial communities. Ecosphere 9:e02090. https://doi.org/10.1002/ecs2.2090
Liu D, Keiblinger KM, Leitner S, Wegner U, Zimmermann M, Fuchs S, Lassek C, Riedel K, Zechmeister-Boltenstern S (2019) Response of microbial communities and their metabolic functions to drying-rewetting stress in a temperate forest soil. Microorganisms 7:129. https://doi.org/10.3390/microorganisms7050129
Meisner A, Rousk J, Baath E (2015) Prolonged drought changes the bacterial growth response to rewetting. Soil Biol Biochem 88:314–322. https://doi.org/10.1016/j.soilbio.2015.06.002
Meisner A, Jacquiod S, Snoek BL, ten Hooven FC, van der Putten WH (2018) Drought legacy effects on the composition of soil fungal and prokaryote communities. Front Microbiol Mar 7(9):294. https://doi.org/10.3389/fmicb.2018.00294
Mikha MM, Rice CW, Milliken GA (2005) Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol Biochem 37:339–347. https://doi.org/10.1016/j.soilbio.2004.08.003
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous determination of nitrate and nitrite. Nitric Oxide 5:62–71
Moore JM, Klose S, Tabatabai MA (2000) Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol Fertil Soils 31:200–210
Najera F, Dippold MA, Boy J, Seguel O, Koester M, Stock S, Merino C, Kuzyakov Y, Matus F (2020) Effects of drying/rewetting on soil aggregate dynamics and implications for organic matter turnover. Biol Fertil Soils 56:893–905. https://doi.org/10.1007/s00374-020-01469-6
Or D, Smets BF, Wraith JM, Dechesne A, Friedman SP (2007) Physical constraints affecting bacterial habitats and activity in unsaturated porous media – a review. Adv Water Res 30:1505–1527. https://doi.org/10.1016/j.advwatres.2006.05.025
Parker SS, Schimel JP (2011) Soil nitrogen availability and transformations differ between the summer and the growing season in a California grassland. Appl Soil Ecol 48:185–192. https://doi.org/10.1016/j.apsoil.2011.03.007
Patel KF, Myers-Pigg A, Bond-Lamberty B, Fansler SJ, Norris CG, McKever SA, Zheng JQ, Rod KA, Bailey VL (2021) Soil carbon dynamics during drying vs. rewetting: importance of antecedent moisture conditions. Soil Biol Biochem 156:108165. https://doi.org/10.1016/j.soilbio.2021.108165
Rayment G, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press Pty Ltd. Melbourne
Rey A, Oyonarte C, Morán-López T, Raimundo J, Pegoraro E (2017) Changes in soil moisture predict soil carbon losses upon rewetting in a perennial semiarid steppe in SE Spain. Geoderma 287:135–146. https://doi.org/10.1016/j.geoderma.2016.06.025
Schaeffer SM, Homyak PM, Boot CM, Roux-Michollet D, Schimel JP (2017) Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biol Biochem 115:54–62. https://doi.org/10.1016/j.soilbio.2017.08.009
Setia R, Marschner P, Baldock JA, Chittleborough D, Smith P, Smith J (2011) Salinity effects on carbon mineralization in soils of varying texture. Soil Biol Biochem 43:1908–1916
Slessarev EW, Lin Y, Jimenez BY, Homyak PM, Chadwick OA, D’Antonio CM, Schimel J (2020) Cellular and extracellular C contributions to respiration after wetting dry soil. Biogeochemistry 147:307–324
Stark JM, Firestone MK (1995) Mechanisms for soil-moisture effects on activity of nitrifying bacteria. Appl Env Microbiol 61:218–221
Tiemann LK, Billings SA (2011) Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol Biochem 43:1837–1847. https://doi.org/10.1016/j.soilbio.2011.04.020
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Warren CR (2014) Response of osmolytes in soil to drying and rewetting. Soil Biol Biochem 70:22–32. https://doi.org/10.1016/j.soilbio.2013.12.008
Wilke B-M (2005) Determination of chemical and physical soil properties. In: R Margesin, F Schinner (Eds) Monitoring and assessing soil bioremediation. Springer, pp 47–95
Willis RB, Montgomery ME, Allen PR (1996) Improved method for manual, colorimetric determination of total Kjeldahl nitrogen using salicylate. J Agric Food Chem 44:1804–1807
Wu J, Brookes PC (2005) The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol Biochem 37:507–515. https://doi.org/10.1016/j.soilbio.2004.07.043
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. KOE is supported by a grant from the Ekhagastiftelsen (Ekhaga Foundation) (grant 2019–16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erinle, K.O., Marschner, P. Time between two partial rewetting events influences the respiration flush and microbial growth after the final rewetting. Biol Fertil Soils 58, 541–552 (2022). https://doi.org/10.1007/s00374-022-01642-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01642-z