Abstract
This position paper summarizes the current understanding of biological nitrification inhibition (BNI) to identify research needs for accelerating the development of BNI as a N2O mitigation strategy for grazed livestock systems. We propose that the initial research focus should be on the systematic screening of agronomically desirable plants for their BNI potency and N2O reduction potential. This requires the development of in situ screening methods that can be combined with reliable N2O emission measurements and microbial and metabolomic analyses to confirm the selective inhibition of nitrification. As BNI-induced reductions in N2O emissions can occur by directly inhibiting nitrification, or via indirect effects on other N transformations, it is also important to measure gross N transformation rates to disentangle these direct and indirect effects. However, an equally important challenge will be to discern the apparent influence of soil N fertility status on the release of BNIs, particularly for more intensively managed grazing systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is a powerful greenhouse gas (GHG) with a global warming potential close to 300 times that of carbon dioxide. Globally, agriculture contributes around 52% of anthropogenic N2O emissions, with animal urine patches the largest N2O source in grazed livestock systems (Tian et al. 2020). The inhibition of soil nitrification, which is the conversion of ammonium to nitrate, has been shown to reduce N2O emissions, with much of the existing understanding of the abatement potential based on studies using synthetic nitrification inhibitors (SNIs; de Klein et al. 2011; Di and Cameron 2016; Minet et al. 2016a; Chadwick et al. 2018). However, there is increasing evidence that plant-induced biological nitrification inhibition (BNI), defined here as attenuation of the nitrification process resulting from the introduction of plant secondary metabolites into the soil through root exudation or turnover of plant tissue, can also reduce N2O emissions (Subbarao et al. 2013; Byrnes et al. 2017; Villegas et al. 2020). Although SNIs provide the flexibility to target applications at specific times, locations, and doses to maximize N2O reduction, BNIs offer other advantages such as (i) a root delivery network reaching into nitrifying sites in the soil; (ii) affecting both ammonia mono-oxygenase (AMO) and hydroxylamine oxidoreductase (HAO), two enzymes involved in nitrification (compared to SNI which only acts on the AMO pathway); (iii) not requiring synthetic production and mechanical application and therefore potentially lowering costs; (iv) potential for continuous formation in growing plants; and (v) being more natural, thus offering the potential for greater public acceptance. On the other hand, the effectiveness of BNI relies on soil incorporation of plant tissue containing BNI compounds, or the rhizodeposition of BNI active compounds into the soil. The latter is modulated by many plant-soil interactions and as yet poorly understood (Nardi et al. 2020). We acknowledge that SNI and BNI could be complementary as N2O mitigation options for grazed livestock systems, but we focus here specifically on the potential of BNIs and their development as a recognized N2O mitigation strategy for livestock systems. The following sections summarize our current understanding and identify key research needs for accelerating this development along key stages of the innovation pipeline (Fig. 1):
-
(1)
Identifying candidate forage species with the genetic capacity to synthesize BNI compounds (discovery)
-
(2)
Maximizing the BNI capacity of these compounds in soils with agronomically viable species (proof of concept)
-
(3)
Managing species within systems to maintain BNI effect and productivity (proof of function)
-
(4)
Implementing systems to incentivize farmers to adopt BNI as a N2O mitigation strategy (recognized mitigation option)
Discovery: which source-plants have the genetic capacity to regulate BNI?
Much of the work to date has focused on (sub)tropical systems and common agricultural plants that have been shown to exhibit the BNI trait naturally including Brachiaria humidicola (syn. Urochloa), wheat, sorghum, maize, rice (Subbarao and Searchinger 2021), and Elymus grass (Li et al. 2022). There is some evidence that the temperate forb species plantain (Plantago lanceolata) may also exhibit BNI effects (Judson et al. 2019). For all these species, BNI-active root exudates have been identified and many of these plants have genetic variation in BNI capacity among wild populations and modern cultivars (Navarrete et al. 2016; Nardi et al. 2020; Subbarao et al. 2021). Recent research has also demonstrated that the BNI trait from the wild grass Leymus racemosus can be successfully transferred via inter-specific hybridization into elite wheat cultivars without disrupting agronomic features or using regulated gene technologies (Subbarao et al. 2021). Therefore, key elements of success at the “discovery” stage are that high potency source-plants containing the BNI trait are identified and that interventions to transfer the trait from potentially raw germplasm sources into elite forage cultivars can occur within agronomic constraints. These efforts will benefit from deciphering the fundamental genetic control of BNI traits in source plants, knowledge of the candidate genes influencing BNI trait expression, and highly efficient means of screening for BNI expression in candidate source populations and large-scale breeding populations.
Proof of concept: what is the N2O reduction potential and how can the BNI effect be maximized?
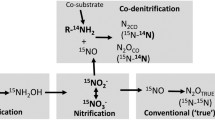
The reduction potential of N2O emissions through BNI depends on the microbial community composition, abundance, and activity of nitrifiers. The common understanding is that N2O originating from nitrification is largely produced by ammonia oxidizing bacteria (AOB) and much less so by ammonia oxidizing archaea (AOA) (Prosser et al. 2020). However, a recent study with pure cultures of the AOA Nitrosopumilus maritimus showed that this AOA can also produce N2O from nitrification (Kraft et al. 2022). These authors showed that under the anaerobic conditions of the study, the AOA was capable of generating and re-using oxygen (O2) to support their metabolic activity. This suggests that AOA can perform nitrification and produce N2O under anaerobic conditions. However, the implications of this finding for managed livestock systems requires further investigation, as increased N availability in these systems is likely to favor AOB over AOA (Egenolf et al. 2022). In addition, the relative contribution of AOA vs AOB to nitrification in different ecosystems is not fully understood yet.
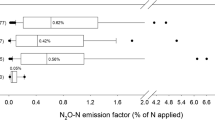
N2O reductions due to BNI have been measured for some key tropical and subtropical grass species, including Brachiaria humidicola and Guinea grass (Megathyrsus maximus) (Subbarao et al. 2013; Byrnes et al. 2017; Villegas et al. 2020). Byrnes et al. (2017) showed that soils containing a Brachiaria cultivar with high BNI capacity emitted 60% less N2O from urine patches than soils with low BNI capacity cultivars, and Villegas et al. (2020) identified varieties of Guinea grass with high N2O reduction potentials. Both studies found a direct link between N2O reduction and BNI, i.e., reduced nitrifier bacteria abundance and nitrification rates. In temperate climate systems, plantain (Plantago lanceolata) has been suggested as a species with BNI activity (de Klein et al. 2020), but comprehensive investigation into a direct link between N2O reduction and BNI activity is lacking. Furthermore, experimental results on the effect of plantain on N2O emissions from livestock urine are inconsistent, with both reductions and increases in N2O observed (Luo et al. 2018; Simon et al. 2019; Pijlman et al. 2020; Bracken et al. 2021). It is commonly accepted that BNI is an adaptive mechanism that plants use to conserve mineral nitrogen (N) in soils where the competition between plants and microbes for limited N is high. So, one hypothesis is that the inconsistency in the results from intensively managed systems could be attributable to variation in soil N fertility status, with high soil N fertility possibly downregulating the expression of the BNI trait, and thus, the N2O reduction potential. A recent study indeed suggested that while BNI seems to determine net nitrification rates in extensive pasture systems with B. humidicola, inter- and intra-competition for N between microbes and plants appeared to be the main determinant in intensive systems (Egenolf et al. 2022). However, the impact of soil N fertility status on BNI-trait expression is yet to be systematically investigated; more studies into this effect are needed. This should include experiments under controlled conditions in greenhouses that enable a focus on specific controlling factors, as well as field trials to investigate the impact under grazing conditions. There are also other potential factors that regulate the release of BNI compounds, including soil pH, soil moisture content, soil aeration, and nematode activity (Wurst et al. 2010; Zhang et al. 2022), that warrant systematic testing in field studies. As it is difficult to separate BNI effects from other plant effects on soil N transformations and microbial community, such studies should measure gross N transformation rates to disentangle the direct and indirect effects of root exudates on soil nitrification (Nardi et al. 2020; Ma et al. 2021). In addition, studies should combine N2O measurements with metabolomics and microbial analysis to confirm both the release of BNI compounds as well as nitrifier inhibition of nitrification, thus directly linking any reduction in N2O emissions with BNI under field conditions.
Proof of function: how can the BNI trait for N2O reduction be optimized in grazed systems?
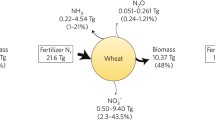
Once our understanding of the links between BNI-induced N2O reduction and soil N status or other regulators is improved, the question is how this can be optimized in grazed livestock systems? This may be especially relevant in legume-containing pasture systems, where there is a strong interaction between soil N fertility status and legume content of the sward, or in grazed systems, where urine deposition results in localized rapid increases in soil N and soil pH. Furthermore, to meet improved productivity as well as environmental outcomes, enhancement of the BNI trait should not compromise the viability of the system through unintended consequences on agronomic characteristics of the species such as productivity, palatability, nutritional value, persistence, winter hardiness, and drought resilience. To date, there is no evidence to suggest that the BNI-trait has a yield penalty either in pastures or in grain crops when comparing BNI-capable varieties with non-BNI capable varieties of the same species (Subbarao and Searchinger 2021). In addition, a BNI-induced increase in farm N use efficiency (NUE) provides the opportunity to reduce farm N inputs and any associated N2O emissions. A recent LCA modeling study suggested that the impacts from BNI-wheat with 40% nitrification inhibition by 2050 could reduce both N fertilizer requirements and GHG emissions by about 15%, and improve NUE at the farm scale by almost 17% (Leon et al. 2021). However, there is limited research on the effects of BNI species on soil, rumen and farm level N cycling in grazed systems, which severely limits our ability to assess the full impact of BNI species on farm scale GHG emissions. More specifically, due to the apparent inverse relationship between soil N fertility and BNI, a key question is whether there is a “sweet spot” of N fertility in managed grazed livestock systems: one that supplies N sufficient to promote exudation of BNI compounds and thus conserve N, yet not too low that plant production is significantly compromised? Another key question is if, and how, the release of BNI compounds is affected by transient changes in soil N and pH in urine patches in grazed systems? In addition, the effect of grazing intensity on soil aeration and root exudation (Sun et al. 2017), and their subsequent impacts on microbial community composition and function, also warrant further investigation. Finally, for optimizing BNIs within grazed systems there may be advantages in synthesizing “BNI active” plant compounds that are delivered to the soil via surface application or in animal feeds (Minet et al. 2016b). Although this would eliminate some advantages of BNIs over SNIs, as discussed above, it could provide a solution in the shorter-term, whilst longer-term plant screening and breeding programs are developed and root-delivery of BNI compounds is maximized.
Recognized mitigation option: how can farmers be recognized for BNI-induced N2O reduction in grazing systems?
For farmers to be recognized for achieving N2O reductions, the effect of the intervention on total N2O emissions needs to be accounted for in GHG inventory methodologies and on-farm accounting tools. To the best of our knowledge, BNI is not (yet) recognized as a N2O mitigation technology in national GHG inventories nor in on-farm accounting tools. This not only requires robust evidence of the efficacy of BNI and the ability to predict N2O reductions under a range of temporal and spatially variable conditions, but it also requires the ability to accurately estimate and record the BNI “activity” of plants. For BNI-active plants in grazed systems, this means being able to demonstrate and verify the effects of their presence in the swards and the conditions that influence their efficacy in N2O reduction.
Conclusions
For BNI to be successfully exploited as a N2O mitigation option in grazed livestock systems, we identified key questions along key stages of the innovation pipeline and possible approaches to address these (Table 1). We propose that the initial research focus should be prioritized on the “discovery” and “proof of concept” stages. Firstly, the systematic screening of agronomically desirable plants and cultivars to identify their ability to synthesize and exude BNI compounds (i.e., do these plants have the genetic blueprint for BNI?) requires the development of in situ screening methods that can be combined with reliable N2O emission measurements as well as measurements of gross N transformation rates. To ensure that any N2O reduction can be assigned to BNI, these measurements should also be accompanied by microbial and metabolomic analyses to confirm the selective inhibition of nitrification. Secondly, whilst understanding the genetic regulation of BNI is a key first step, an equally important challenge will be to discern the apparent influence of soil N fertility status or other soil and climatic factors on the release of the BNIs, particularly for more intensively managed grazing systems. The expansion of an existing BNI consortium (Subbarao and Searchinger 2021) to develop a coordinated global program to address the research gaps we identified here may be a key step towards accelerating the development of BNI as a N2O mitigation option in both (sub)tropical and temperate livestock systems.
References
Bracken CJ, Lanigan GJ, Richards KG, Müller C, Tracy SR, Grant J, Krol DJ, Sheridan H, Lynch MB, Grace C, Fritch R, Murphy PNC (2021) Source partitioning using N2O isotopomers and soil WFPS to establish dominant N2O production pathways from different pasture sward compositions. Sci Total Environ 781. https://doi.org/10.1016/j.scitotenv.2021.146515
Byrnes RC, Nùñez J, Arenas L, Rao I, Trujillo C, Alvarez C, Arango J, Rasche F, Chirinda N (2017) Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol Biochem 107:156–163
Chadwick DR, Cardenas LM, Dhanoa MS, Donovan N, Misselbrook T, Williams JR, Thorman RE, McGeough KL, Watson CJ, Bell M, Anthony SG, Rees RM (2018) The contribution of cattle urine and dung to nitrous oxide emissions: quantification of country specific emission factors and implications for national inventories. Sci Total Environ 635:607–617
de Klein CAM, Cameron KC, Di HJ, Rys G, Monaghan RM, Sherlock RR (2011) Repeated annual use of the nitrification inhibitor dicyandiamide (DCD) does not alter its effectiveness in reducing N2O emissions from cow urine. Anim Feed Sci Technol 166–167:480–491
de Klein CAM, van der Weerden TJ, Luo J, Cameron KC, Di HJ (2020) A review of plant options for mitigating nitrous oxide emissions from pasture-based systems. N Z J Agric Res 63:29–43
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sed 16:1401–1420
Egenolf K, Schad P, Arevalo A, Villegas D, Arango J, Karwat H, Cadisch G, Rasche F (2022) Inter-microbial competition for N and plant NO3− uptake rather than BNI determines soil net nitrification under intensively managed Brachiaria humidicola. Biol Fertil Soils (this issue)
Judson HG, Fraser PM, Peterson ME (2019) Nitrification inhibition by urine from cattle consuming Plantago lanceolata. J N Z Grasslands 81:111–116
Kraft B, Jehmlich N, Larsen M, Bristow LA, Könneke M, Thamdrup B, Canfield DE (2022) Oxygen and nitrogen production by an ammonia-oxidizing archaeon. Science 375:97–100
Leon A, Subbarao GV, Kishii M, Matsumoto N, Kruseman G (2021) An ex ante life cycle assessment of wheat with high biological nitrification inhibition capacity. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-16132-2
Li W, Ma J, Bowatte S, Hoogendoorn C, Hou F (2022) Evidence of differences in nitrous oxide emissions and biological nitrification inhibition among Elymus grass species. Biol Fertil Soils (this issue)
Luo J, Balvert SF, Wise B, Welten B, Ledgard SF, de Klein CAM, Lindsey S, Judge A (2018) Using alternative forage species to reduce emissions of the greenhouse gas nitrous oxide from cattle urine deposited onto soil. Sci Total Environ 610–611:1271–1280
Ma Y, Jones DL, Wang J, Cardenas LM, Chadwick DR (2021) Relative efficacy and stability of biological and synthetic nitrification inhibitors in a highly nitrifying soil: evidence of apparent nitrification inhibition by linoleic acid and linolenic acid. Eur J Soil Sci 72:2356–2371
Minet EP, Jahangir MMR, Krol DJ, Rochford N, Fenton O, Rooney D, Lanigan G, Forrestal PJ, Breslin C, Richards KG (2016a) Amendment of cattle slurry with the nitrification inhibitor dicyandiamide during storage: a new effective and practical N2O mitigation measure for landspreading. Agric Ecosyst Environ 215:68–75
Minet EP, Ledgard SF, Lanigan GJ, Murphy JB, Grant J, Hennessy D, Lewis E, Forrestal P, Richards KG (2016b) Mixing dicyandiamide (DCD) with supplementary feeds for cattle: an effective method to deliver a nitrification inhibitor in urine patches. Agric Ecosyst Environ 231:114–121
Nardi P, Laanbroek HJ, Nicol GW, Renella G, Cardinale M, Pietramellara G, Weckwerth W, Trinchera A, Ghatak A, Nannipieri P (2020) Biological nitrification inhibition in the rhizosphere: determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol Rev 44:874–908
Navarrete S, Kemp PD, Pain SJ, Back PJ (2016) Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Anim Feed Sci Technol 222:158–167
Pijlman J, Berger SJ, Lexmond F, Bloem J, van Groenigen JW, Visser EJW, Erisman JW, van Eekeren N (2020) Can the presence of plantain (Plantago lanceolata L.) improve nitrogen cycling of dairy grassland systems on peat soils? N Z J Agric Res 63:106–122
Prosser JI, Hink L, Gubry-Rangin C, Nicol GW (2020) Nitrous oxide production by ammonia oxidizers: physiological diversity, niche differentiation and potential mitigation strategies. Glob Chang Biol 26:103–118
Simon PL, de Klein CAM, Worth W, Rutherford AJ, Dieckow J (2019) The efficacy of Plantago lanceolata for mitigating nitrous oxide emissions from cattle urine patches. Sci Total Environ 691:430–441
Subbarao GV, Searchinger TD (2021) A “more ammonium solution” to mitigate nitrogen pollution and boost crop yields. Proc Natl Acad Sci USA 118. https://doi.org/10.1073/pnas.2107576118
Subbarao GV, Rao IM, Nakahara K, Sahrawat KL, Ando Y, Kawashima T (2013) Potential for biological nitrification inhibition to reduce nitrification and N2O emissions in pasture crop-livestock systems. Animal 7(Suppl 2):322–332
Subbarao GV, Kishii M, Bozal-Leorri A, Ortiz-Monasterio I, Gao X, Ibba MI, Karwat H, Gonzalez-Moro MB, Gonzalez-Murua C, Yoshihashi T, Tobita S, Kommerell V, Braun HJ, Iwanaga M (2021) Enlisting wild grass genes to combat nitrification in wheat farming: a nature-based solution. Proc Natl Acad Sci USA 118. https://doi.org/10.1073/pnas.2106595118
Sun G, Zhu-Barker X, Chen D, Liu L, Zhang N, Shi C, He L, Lei Y (2017) Responses of root exudation and nutrient cycling to grazing intensities and recovery practices in an alpine meadow: an implication for pasture management. Plant Soil 416:515–525
Tian H, Xu R, Canadell JG, Thompson RL, Winiwarte W, Suntharalingam P, Davidson EA, Ciais P, Jackson RB, Janssens-Maenhout G, Prathe MJ, Regnier P, Pan N, Pan S, Peters GP, Shi H, Tubiello FN, Zaehle S, Zhou F, Arneth A, Battaglia G, Berthet S, Bopp L, Bouwman AF, Buitenhuis ET, Chang J, Chipperfield MP, Dangal SRS, Dlugokencky E, Elkins JW, Eyre BD, Fu B, Hall B, Ito A, Joos F, Krummel PB, Landolfi A, Laruelle GG, Lauerwald R, Li W, Lienert S, Maavara T, MacLeod M, Millet DB, Olin S, Patra PK, Prinn RG, Raymond PA, Ruiz DJ, van der Werf GR, Vuichard N, Wang J, Weiss RF, Wells KC, Wilson C, Yang J, Yao Y (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256
Villegas D, Arevalo A, Nuñez J, Mazabel J, Subbarao G, Rao I, De Vega J, Arango J (2020) Biological nitrification inhibition (BNI): phenotyping of a core germplasm collection of the tropical forage grass Megathyrsus maximus under greenhouse conditions. Front Plant Sci 11: article 820. https://doi.org/10.3389/fpls.2020.00820
Wurst S, Wagenaar R, Biere A, van der Putten WH (2010) Microorganisms and nematodes increase levels of secondary metabolites in roots and root exudates of Plantago lanceolata. Plant Soil 329:117–126
Zhang M, Zeng H, Afzal MR, Gao X, Li Y, Subbarao GV, Zhu Y (2022) BNI-release mechanisms in plant root systems: current status of understanding. Biol Fertil Soils (this issue)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This project was part-funded by the New Zealand Government, in support of the objectives of the Livestock Research Group of the Global Research Alliance on Agricultural Greenhouse Gases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Klein, C.A.M., Bowatte, S., Simon, P.L. et al. Accelerating the development of biological nitrification inhibition as a viable nitrous oxide mitigation strategy in grazed livestock systems. Biol Fertil Soils 58, 235–240 (2022). https://doi.org/10.1007/s00374-022-01631-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01631-2