Abstract
Plant residues with larger carbon (C) to nitrogen (N) ratios can stimulate microbial growth and thereby protect soil nutrients from leaching. In poorly fertilized soil, excessive immobilization may limit nutrient availability and thus plant growth. Little is known about the impact of a shallow straw incorporation on soil microbial regulation of top-dressing fertilizer nutrients and spring crop establishment. We aimed to evaluate if wheat straw in combination with mineral fertilizer has more positive effects on plant performance than mineral fertilization alone and if this relates to changes of the extractable C:N:P ratio and microbial activity close to the roots. In order to conduct small-scale sampling with minimal disturbance during growth of spring barley (Hordeum vulgare L.), we developed rhizotrons with resealable ports. Rhizotrons were filled with loamy-sandy soil and fertilized with an equivalent of 150 kg N and 80 kg P ha−1. Half of the rhizotrons received the top dressing together with 4500 kg wheat straw-C ha−1. Throughout a 90-day greenhouse experiment, we analyzed soil C:N:P dynamics, and carbon dioxide (CO2) and nitrous oxide (N2O) emission, together with microbial biomass, selected bacterial genes (abundance), and transcripts (activity) in bulk and root-affected soil at multiple times. We focused on nitrifiers and denitrifiers and linked our data to barley growth. Interactions between straw and roots caused shifts towards larger C:P and C:N ratios in root-affected soil. These shifts were associated with increased 16S rRNA transcripts and denitrifier activities. Straw increased microbial biomass by 124% in the topsoil and at the same time increased root biomass by 125% and number of tillers by 80%. We concluded that microbial activation at the root-straw interface may positively feed back on soil nutrient regulation and plant performance. Further research has to evaluate if plant roots actively prime mining of previously immobilized nutrients in the straw detritusphere or if effects of pathogen suppression and growth promotion are dominating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is increasingly recognized that conventional farming practice and mineral fertilization have negative impact on soil humus stocks (Steinmann et al. 2016) and, less recognized, on microbiological potentials to regulate soil nutrient retention (NO3− leaching, Li et al. 2021) and release to promote plant growth (Malhi et al. 2011). In agricultural soils, due to mineral fertilization, microorganisms are mostly limited by organic C and only secondarily by N or P (Griffiths et al. 2012). Plant and soil microorganisms are assumed to compete for N, if C:N ratios of accessible resources are larger than 25 (Amelung et al. 2018). Usually, C:N ratios in agricultural soils are below this threshold, if not temporarily increased, e.g., by incorporation of cereal straw with wider C:N ratios of 50–100. Such straw residues are also characterized by a wide holocellulose:lignin ratio (Wei et al. 2020), which marks the residues as more accessible and faster decomposable to soil microorganisms. Under such conditions, soil microorganisms gain a temporal competitive advantage over plants for available mineral N for biomass production. Therefore, incorporation of straw before winter could be used as a strategy to immobilize excess mineral N in new microbial biomass (Amelung et al. 2018; Reichel et al. 2018). This protects N from leaching during wet winter periods in a manner similar to catch cropping by temporarily immobilizing available soil nutrients in new biomass (Li et al. 2021). Next year’s crops may benefit from the straw-derived microbial N sources, when straw decomposition, and thus C release, subsides and the previously straw-mediated microbial matter is remineralized (Reichel et al. 2018). Organic C oversupply and decay-limiting factors, e.g., high lignin contents as in sawdust, could prolong nutrient immobilization into the next growing season, resulting in critical “N lock” and reduced crop yield (van Duijnen et al. 2018).
Co-application of mineral N and P with straw, however, mitigates “N lock” conditions and additionally favors a more diverse and complex microbial community (Zhao et al. 2019), containing more bacterial and fungal taxa related to C- and N-cycling than in soil without straw amendment (Zhong et al. 2020). Mycorrhizal fungi are plant symbionts and saprotrophic microorganism with the capability to penetrate and take up N from organic patches (Hodge et al. 2001). Yang et al. (2019) reported that bacterial taxa living in the straw detritusphere could favor N availability to plants. They further showed a decrease in plant pathogens with straw amendment as compared to the un-amended control. Suppression of pathogens such as Fusarium wilt in watermelon orchards can be related to positive impacts of wheat straw on the rhizosphere microbial network and diversification of the rhizospheric microbiome (Tang et al. 2020). Straw addition may also prime the disease defenses by an improved uptake of silica from the detritusphere into plant biomass (Rodgers-Gray and Shaw 2000). Overall, N and P fertilization in combination with wheat straw incorporation at a rate of 3.75 t ha−1 a−1 prior to plowing significantly increased soil organic C, total N, and microbial biomass to which crop yields responded positively (Liu et al. 2010).
However, literature is not conclusive about the effects of a shallow straw detritusphere on microbial regulation of top-dressing fertilizer nutrients and on plant performance. Therefore, and in order to conduct small-scale sampling of soil with minimal disturbance on overall soil C and N dynamics, we developed special rhizotrons with resealable ports.
Generally, rhizotron experiments with plant roots growing along a transparent 2D surface are important to improve the mechanistic understanding of dynamic effects, especially if soil and root samplings at a fine temporal and spatial scale can be ensured without massive disturbance or disintegration of the whole soil system. Many methods have been developed to study the root architecture, dynamics, regulatory, and physicochemical mechanisms for mechanistic understanding and modeling to develop sustainable plant management strategies (Hilton et al. 1969; Neumann et al. 2009). More recently, informatics and robotics have led to the invention of automated phenotyping systems such as the GROWSCREEN-Rhizo, which is capable of imaging plant roots and shoots of 60 soil-filled rhizotrons per hour (Nagel et al. 2012). However, most of the rhizotrons were not designed to allow flexible soil sampling or manipulations, e.g., of nutrients or microbial activity, at basically any spot and time during the observation of plant and root development, even if state-of-the-art techniques such as X-ray tomography, magnetic resonance imaging, light transmission imaging, or neutron radiography are applied (Lobet and Draye 2013). Other authors used rather artificial setups with nylon cloth or plates with fine open holes to differentiate technically between different soil and root compartments or to insert micro-suction cups to extract nutrients and other compounds (Kuchenbuch and Jungk 1982; Youssef and Chino 1987; Dessureault-Rompré et al. 2006). In the frame of this study, we used a custom-made rhizotron system with re-sealable sampling ports for a more spatially and temporally flexible soil sampling over time, including different soil depths and soil compartments without substantial disturbance of the plant-soil-microbial system. We used the new rhizotrons to conduct a 90-day greenhouse experiment in which we analyzed soil C:N:P dynamics, carbon dioxide (CO2), and nitrous oxide (N2O) emissions, together with microbial biomass, selected bacterial genes (abundance), and transcripts (activity) in bulk and root-affected soil at multiple times. Our experiment was guided by two hypotheses: (1) shallow wheat straw incorporation in combination with top-dressing mineral fertilization has a more positive effect on plant performance than mineral fertilization alone; (2) positive effects on plant performance can be related to shifts in the extractable C:N:P ratio and increased microbial activity close to the fertilizer-enriched straw detritusphere.
Material and methods
Experimental design
Eight of the specially designed rhizotrons were used (Fig. 1). Details about the rhizotron design and the sampling technique are provided in the supplementary material (Fig. S1a, b, c).
Custom-made rhizotron with three segments and re-sealable sampling openings. Each rhizotron segment had an inner dimension of (D × W × H) 25 mm × 350 mm × 500 mm (4.4 L volume), containing a grid at both sides with 520 sampling openings (Ø 8 mm). Fifteen resealable openings at the side struts provide access to insert further tools. Four openings at the bottom help to collect leachate water. The number of segments and angle of the rhizotrons can be adjusted to the required experimental conditions
A realistic soil profile with a sequence of an A, B, and C horizon was simulated by filling three different soil substrates stepwise into the rhizotrons. Each substrate layer was compacted to the specified bulk density by defined rubber hammer strokes (Table S1). On average, each rhizotron contained 13.3 kg dry substrate with a standard deviation (± SD) between replicates (n = 4) of 1.1%. Soil substrate 1 was obtained as freshly sampled substrate, ordered as “standard soil 2.1” from the Agricultural Investigation and Research Institute LUFA (Speyer, Germany) and used to fill the upper 28 cm of the rhizotron. It was derived from a fallow agricultural Ap horizon without any fertilization or pesticide application during the past 4 years. The soil texture was characterized as loamy sand with 4% clay, 10% silt, and 86% sand. Soil substrate 2 was composed of substrate 1, quartz powder (Ø particle size 90 µm), and quartz sand (Ø particle size 370 µm), mixed at a volumetric ratio of 1:1:6 to form the artificial B horizon at a depth of 28 to 85 cm. Soil substrate 3 contained only quartz sand with an average grain size of 370 µm, forming the C horizon at a depth of 85 to 100 cm. More details about the properties of the substrates can be found in Table S1.
Soil moisture content was slowly adjusted to 30% of the average WHC. The rhizotrons were covered with PARAFILM ® M (VWR International GmbH, Darmstadt, Germany) to reduce evaporation and were pre-incubated more than 1 month (8 weeks) to allow for the stabilization of the soil microbial C and N dynamics after rewetting the soil (Fierer and Schimel 2002). Afterwards, the eight rhizotrons were split randomly into a control (mineral fertilizer only, NP) and a wheat straw treatment (mineral fertilizer plus wheat straw, WSNP) group, each comprising four independent replicates.
The practically relevant plant density and fertilizer amount were calculated based on the rhizotron soil surface of 0.00875 m2. Each rhizotron received 0.56 g ± 1.1% N fertilizer (YaraBela® SULFAN™: 12% NO3−-N, 12% NH4+-N, 6% S; 12% CaO; YARA GmbH and Co. KG, Dülmen, Germany) and 0.36 g ± 1.1% triple superphosphate (46% P2O5 = 20% P; Raiffeisen Waren-Zentrale Rhein-Main eG, Köln, Germany), equivalent to 150 kg N ha−1 and 80 kg P ha−1. Fertilizer grains were evenly distributed at the soil surface, with a distance of 30 mm from each other. The expected N immobilization maximum of 40 kg N ha−1 after wheat straw incorporation in the first month after incorporation was used (Reichel et al. 2018) to adjust the N fertilization to a non-limiting level. In addition, half of the rhizotrons received an equivalent of 4500 kg C ha−1 using 2–5 mm otherwise untreated wheat straw particles (4.0 g per rhizotron, SD ± 0.03%), mixed into the top 0–2 cm of the soil (shallow wheat straw incorporation zone) to manipulate the stoichiometric ratio of C:N:P in this layer.
In contrast to other studies with straw incorporation into the entire plowing layer, we used a shallow incorporation of straw to manipulate the C:N:P stoichiometry close to the mineral fertilizer application zone, which usually is also the soil volume with the largest microbial biomass and activity potential to regulate soil nutrients. Air-dry wheat straw was obtained in 2017 from the Hohenschulen experimental site (Kiel, Germany) and stored under dry conditions at room temperature. The wheat straw had the following characteristics: C = 44.4 ± 0.1%, N = 0.28 ± 0.02%. Two pre-grown summer barley plants (Hordeum vulgare L. cv. LEANDRA zz Brau, Hauptsaaten für die Rheinprovinz GmbH, Germany), corresponding to a density of 228 seed grains per m2, were planted at radicle emergence stage. To eliminate previously reported negative effects of wheat straw on seed germination, e.g., from toxins and pathogenic fungi (Kimber 1973; Nakano et al. 2006; Raaijmakers et al. 2009), barley seedlings were transplanted 20 days after straw application (20 DAF), when most critical straw effects, e.g., of pathogens, most likely had subsided.
The rhizotrons were randomly shifted after each sampling, and the mean of both plants per rhizotron was used for later calculations.The experiment was conducted from January 25 to April 10, 2019, in a greenhouse of the Forschungszentrum Jülich GmbH (Germany; 50°54′31.9″N 6°24′11.0″E). Air temperature in the greenhouse was not controlled and ranged between 1 °C and 18 °C (Fig. S2). A temperature logger with an external sensor was placed in a shaded area near the soil surface to record the air temperature hourly. Relative humidity ranged between 50 and 70% during the experiment (Fig. S2) and was recorded regularly at 12 a.m., using a standard hygrometer. Artificial lighting (SANLight S2W LED lights, SANlight GmbH, Bludenz, Germany) was installed 100 cm above each rhizotron with a spectrum of 400 to 730 nm and a light intensity of 200 µmol m−2 s−1 14.5 h daily. The rhizotrons were watered with 60 mL unsterile, deionized water (spiked with 0.003 M CaCO3) twice per week. Soil moisture was allowed to fluctuate based on evapotranspiration changes.
Soil sampling
Sampling comprised four independent replicates per treatment. Per replicate, two separate soil samples of 1 cm3 with an average fresh weight of 1.5 g each were sampled. These two separate samples were pooled and then split again. One half was stored at 4 °C for gas emission analysis and extraction of soil nutrients, the other half was used as backup or for molecularbiological analyses (− 80 °C). Samples from different soil depths were taken at different vertical, but a fixed horizontal location. Samples of bulk soil and root-affected soil were sampled from a fixed vertical, but from different horizontal locations. For the determination of microbial biomass C and N, we destructively sampled the soil at the end of the experiment, as described in more detail below.
To evaluate time effects of a shallow incorporation of wheat straw on distribution of top-dressing fertilizer nutrients, samples were collected from bulk soil 3–5 cm below the treatment application zone 0, 6, 20, 40, 49, and 84 DAF (days after wheat straw amendment and fertilization). Uppermost soil centimeters were omitted to allow an even distribution of dissolving fertilizer along the soil surface and the straw detritusphere.
In order to evaluate the straw effect on the nutrient distribution and N-cycling genes and transcripts in root-affected and bulk soil, sampling was conducted at 3–5-cm soil depth 13 and 29 days after barley transplanting (33 and 49 DAF). Bulk soil was defined as soil from locations without visible roots along the rhizotron window and without hidden roots in the sample. Root-affected soil was defined as soil from locations with visible roots along the rhizotron window. Cutoff roots were removed from root-affected soil before processing. Genes and transcripts of nitrifiers and denitrifiers were assessed at tillering stage 49 DAF (phenological development coding: BBCH 22), which was the earliest time at which visual differentiation between bulk and root-affected soil was possible.
Bulk soil samples were obtained from 3–5 cm, 5–10 cm, 10–20 cm, and 20–30 cm below the soil surface 20 DAF (BBCH 00), 40 DAF (BBCH 22), and 84 DAF (BBCH 33) to evaluate the wheat straw treatment effect on soil C:N:P stoichiometry, and CO2 and N2O gas emission at different soil depths.
Additional bulk soil samples were collected from the soil layers 0–5 cm, 5–10 cm, 10–30 cm (A horizon), and 30–40 cm (B horizon) to quantify the wheat straw effect on microbial biomass C (Cmic) and microbial N (Nmic) at the end of the experiment. These samples were pooled, mixed, and stored at 4 °C until processing.
Soil physicochemical analyses
The following sample processing pipeline for greenhouse gas (GHG) and nutrient analyses was applied.
Step 1: Determining the weight of the fresh soil core in a 22-mL gas chromatograph (GC) vial directly after sampling (crimp neck vial N 20, MACHEREY–NAGEL GmbH and Co. KG, Düren, Germany), followed by gas chromatographic analysis of the GHG after 12 h at 20 °C (GC 8610C with electron capture detector, SRI Instruments, CA, USA). The evaporation protection (PARAFILM ® M, VWR International GmbH, Darmstadt, Germany) was removed 1 h before crimping the vials gas-tight with butyl rubber septa and aluminum caps (crimp closure N 20, septa hardness 50° shore A, MACHEREY–NAGEL GmbH and Co. KG, Düren, Germany). Twenty-milliliter ambient air was injected with a syringe into each sealed GC vial at the beginning to have enough gas volume for repeated sampling by the GC auto-sampler after 1, 4, and 7 h of closed incubation. Ambient air was sampled before the vial preparation as time point zero. Each measurement reduced the gas volume in the vial by 3.5 mL, which was considered by stepwise reduction of the gas volume in the calculation of CO2-C and N2O-N g−1 h−1 (Reichel et al. 2018).
Step 2: After GHG measurement, vials were opened and covered with a perforated aluminum foil, then frozen at − 20 °C, and subsequently freeze-dried. Then, the weight of the dry soil core in the 22-mL GC vial was determined to get dry-to-fresh soil mass ratios for later calculations. Gravimetric water content was determined by dividing the water content by the soil dry mass, multiplied by 100. Freeze-dried samples were stored closed gas-tight at 4 °C until performing the soil extractions as described below.
Step 3: The standard method for soil nutrient extraction (VDLUFA 1991) was modified as follows: nutrient extraction of freeze-dried soil cores was performed in 50-mL falcon tubes (VWR International, Darmstadt, Germany), using 0.01 M CaCl2 solution and a dry soil-to-solution ratio of 1:20 (w/v). Soil samples with an average dry weight of 1.5 g were dispersed in 30 mL of the 0.01 M CaCl2 solution and mixed for 4 h by over-head shaking (Heidolph Reax 2, Heidolph Instruments GmbH and CO. KG, Schwabach, Germany) at a speed of 20 turns min−1. Soil and solution were separated by centrifugation at 690 × g for 30 min. Twenty milliliters of the supernatant was filtered through 0.45-µm PP-membrane filters (disc size 25 mm; Macherey–Nagel, Düren, Germany). The solution was stored at 4 °C until the determination of the pH, dissolved organic C (DOC), mineral N (Nmin = sum of exchangeable NH4+-N and NO3−-N), and 0.01 M CaCl2-extractable phosphorus (PCaCl2). To check the influence of freeze-drying on the compound extractability, a laboratory pre-test was performed on fresh and freeze-dried soil. Results showed that freeze-drying significantly (t test, p < 0.05) increased the DOC content by 109%. The concentration of H+ (pH), Nmin, and PCaCl2 was not significantly affected by freeze-drying of the specific soil. Based on this, only the DOC results after extraction of freeze-dried soil were corrected by a factor of 0.48.
pH values were determined using a pH electrode and a multimeter (multi 340i pH meter, Xylem Analytics Sales GmbH and Co., Weilheim, Germany). Eight milliliters of the fresh soil extract was transferred into a 24-mL screw neck TOC vial (Macherey–Nagel, Düren, Germany) and made up with distilled water to a total volume of 20 mL (dilution factor of 2.5) before analyzing DOC with a TOC analyzer (TOC-VcPH + TNM-1 + ASI-V, Shimadzu, Japan). Twelve milliliters of the final soil extract was transferred into a 20-mL polyethylene vial with a quick closure cap (PerkinElmer Inc., MA, USA). Nitrate and exchangeable NH4+ were measured by ion chromatography (Dionex DX-500, ThermoScientific, MA, USA). Phosphorus (PCaCl2) was determined by inductively coupled plasma optical emission spectrometry (iCAP™ 7600 ICP-OES Analyzer, ThermoScientific, MA, USA).
Leachate was collected if available, frozen at − 20 °C, and combined to a bulk sample to analyze for the nutrients as described above (i.e., step 3).
Microbial analyses
Soil sampled at the end of the experiment from different soil depths was used to determine the microbial biomass C (Cmic) and N (Nmic) by the chloroform-fumigation extraction based on Joergensen (1996). Forty milliliters of the soil extract was centrifuged at 690 × g for 15 min before filtering through a 0.45-µm polypropylene membrane filter (47-mm diameter, Macherey–Nagel, Germany). Ethanol-free chloroform for liquid chromatography was used (LiChrosolv®, Merck Chemicals GmbH, Darmstadt, Germany). The closed desiccator was incubated at room temperature for 24 h. DOC and dissolved N were measured using a TOC analyzer (TOC-VcPH + TNM-1 + ASI-V, Shimadzu, Japan). Cmic and Nmic were calculated as difference of DOC and dissolved N by subtracting non-fumigated blank from fumigated sample and correction by the factors kEC 0.45 and kEN 0.40, as reported by Joergensen (1996).
Abundance and activity of nitrifiers and denitrifiers
DNA and RNA were extracted from 0.5 g of frozen soil (− 80 °C), using a co-extraction protocol modified according to Töwe et al. (2011). The co-extracts were quantified using a Nanodrop 2000 spectrophotometer (ThermoFischer Scientific, USA) and stored at − 80 °C until further analysis. The co-extracts were divided into two tubes and labeled as DNA and RNA for further analysis. DNA digestion was performed on the RNA samples using the RNA Clean and Concentrator-5 kit (ZymoResearch, USA), following the protocol instructions. To assess the success of the DNase treatment, a PCR was performed targeting the V4/V5 region of the 16S rRNA gene. RNA purification was then carried out using the same kit like described above, and the quality and quantity of the purified RNA were determined using the DNF-471 Standard Sensitivity RNA kit on a Fragment Analyzer Instrument (Agilent Technologies, California, USA). cDNA synthesis was performed using the SuperScript IV VILO master mix kit (ThermoFischer Scientific, USA) following the manufacturer’s instructions.
Absolute quantification of the 16S rRNA gene, as well as marker genes for denitrification (nirK, nirS, and nosZ) and nitrification (amoA for ammonia-oxidizing archaea (AOA) and bacteria (AOB)) for both DNA and RNA was done using real-time quantitative PCR (q-PCR) on a 7300 Real-Time PCR System (Applied Biosystems, MA, USA). The primers used are described in Table 1. The PCR reaction mix contained: 1 × Power SYBR™ Green PCR Master Mix (Applied Biosystems, MA, USA), 7.5 pmol of each primer (for the amoA gene of AOB) and 5 pmol of each primer for the other genes, 0.06% bovine serum albumin (BSA), 2.5% DMSO (for nirK and nirS genes), 1 µL of template DNA/cDNA and DEPC water to a final volume of 25 µl. The optimal dilution for the DNA and cDNA was determined by testing for possible inhibitory effects on qPCR amplification caused by co-extracted humic substances. A dilution of (1:100 v/v) of the DNA and cDNA was selected for amplification (data not shown). All PCRs involved an initial activation step at 95 °C for 10 min and 1 cycle of product melting at 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. Melting curves were used to check the dissociation pattern of the amplified products. Serial dilutions (ranging from 101 to 107 gene copies-μl−1) of plasmids containing the tested genes in known concentrations were used to generate the standard curve. The amplification efficiencies were calculated using the formula Eff = [10(−1/slope) − 1] and gene copy numbers normalized to grams of soil.
Plant sampling and analyses
Relative leaf greenness (a proxy for chlorophyll content) was assessed in order to evaluate the wheat straw treatment effect on barley plants as single-photon avalanche diode (SPAD) value 51, 56, 68, 77, and 90 DAF, using a chlorophyll meter (SPAD-502Plus, Konica Minolta, Inc., Tokyo, Japan; based on Süß et al. 2015). To reduce the variability of the SPAD values within each plant and along each leaf, the measurement was performed on the second fully unfolded leaf from the tip of the main stem to estimate the actual chlorophyll status. The instrument was placed at four spots decentral to the leaf primary vein to assess the SPAD median value.
The height of each shoot was measured from the base to the stem below the uppermost main shoot leaf to access the shoot height, leaf number, and tiller number 56, 68, 77, and 90 DAF. Leaf and tiller number of each plant were assessed by manual counting, including all living and dead leaflets.
Root and shoot were separated by cutting the shoot at the base to determine the shoot and root biomass (for roots after thorough washing with tap water) 90 DAF. Plant materials were dried at 60 °C until reaching a constant mass for determining the root and shoot in g plant−1 dm. After ball-milling (MM 400, Retsch, Germany), the total C and N content of the finely ground plant material was measured with an elemental analyzer (Vario EL, Elementar, Germany). Total P content was measured after microwave digestion with 2 mL HNO3 (65%) and 1 mL H2O2 (30%) at 800 W, 20 min gradient from room temperature to 160 °C, and a final holding time of 15 min (MARS 5, CEM GmbH, Kamp-Lintfort, Germany; based on Nischwitz et al. 2017). Digested samples were made up to 14 mL with ultra-pure water. The obtained solution was diluted 1:20 with ultrapure water before measurement with ICP-OES (iCAP™ 7600 ICP-OES Analyzer, ThermoScientific, MA, USA). The results were used to calculate the root-to-shoot and C:N:P ratios (Table S6).
Statistical analyses
Results are presented as mean value ± standard deviation (± SD) of four independent replicates per treatment (control and wheat straw treatment). Statistics were performed using SPSS Statistics 21 (IBM, Ehningen, Germany) and the R software package (V.3.6.0; R Core Team 2020). The data were analyzed using the Shapiro–Wilk test to check if the normality assumption was valid.
One-way analysis of variance (ANOVA) was used to test if straw addition affected plant and microbial parameters. Two-way ANOVA models were used to evaluate the effect of straw addition, time, and their interactions on soil parameters like DOC, Nmin, NO3−, exchangeable NH4+, PCaCl2, CO2, and N2O, as well as on plant parameters such as SPAD, shoot height, and leaf number. Three-way ANOVA models were used to test if straw addition, time, soil depth or root-affected vs. bulk soil, and their interactions affected the soil parameters. Depending on the statistical assumption validity, we performed post hoc comparisons using Tukey-B or t test. Otherwise, the Wilcoxon test, Kruskal–Wallis test, or Mann–Whitney U test was used. More information is presented in corresponding tables or figures.
Results
Prior to the wheat straw and fertilizer application, C:N:P stoichiometry (molar ratio of the parameters of extractable DOC:Nmin:PCaCl2) of the rhizotron soils was statistically not different. Gravimetric moisture content decreased in both treatments over time in the soil layer 3–5 cm (F = 45.9, p < 0.001; Table S2) and showed a declining gradient from top to deeper soil layers (F = 19.2, p < 0.001; Table S3), but no difference between bulk and root-affected soil (Table S4). Bulk leachate samples of the WSNP treatment (88 mL) contained about 14% more DOC (1.2 mg), 3% less Nmin in the form of NO3− (2.8 mg), but 9% more P (2.4 mg) compared to the NP treatment (92 mL).
Dynamics of extractable C:N:P in soil after wheat straw application
In the top soil layer (3–5 cm), time and wheat straw amendment showed interactive effects on the DOC concentration (F = 8.3, p < 0.001; Fig. 2A). In addition, for DOC both factors also interacted with the soil compartment (F = 5.5, p < 0.05; Table S4). Wheat straw incorporation increased the DOC concentration by 79% (p < 0.05) in the WSNP treatment at 6 DAF, followed by a rapid decline to the level of the NP treatment at 20 DAF (Fig. 2A). In the WSNP treatment, DOC concentrations were increased in the root-affected compared to the bulk soil at 49 DAF (Table S4).
Wheat straw effects over time (p < 0.001) on physicochemical soil parameter mean values of the NP (control) and the WSNP (wheat straw) treatment in the soil layer 3–5 cm (below the treatment application zone) zero days after wheat straw amendment and fertilization (0 DAF), 6, 20 (transplanting of barley, black arrow), 40, 49, 84 DAF. (A) Dissolved organic C (DOC), (B) extractable Nmin (exchangeable NH4+-N and NO3−-N), and (C) carbon dioxide emission (CO2 emission). Units are displayed at the y-axis. Regressions with R2 are polynomic. Asterisks indicate significant differences between the treatments at p ≤ 0.05. More details in Table S2
Time (F = 188.4, p < 0.001; Fig. 2), wheat straw treatment (F = 16.8, p < 0.001; Fig. 2), and soil depth (F = 22.2; p < 0.001; Table S3) had an impact on the soil Nmin concentration, while the soil compartment only interacted with the factors time and treatment (F = 10.8, p < 0.01; Table S4). Soil with wheat straw incorporation contained about 25% less Nmin (in the soil layers 3–5 cm: NO3− + exchangeable NH4+; 5–10 cm: NO3−) compared to the NP treatment (Fig. 2B, Tables S2 and S3). In the root-affected soil of the WSNP treatment, 33% less exchangeable NH4+ was observed compared to bulk soil at 49 DAF (Table S4).
Time and the wheat straw amendment had only small effects on extractable PCaCl2 (Table S2) in combination with the factors soil depth (F = 4.2, p < 0.01; Table S3) and soil compartment (F = 4.9, p < 0.05; Table S4). Concentration of PCaCl2 was increased in the 3–5 cm and 5–10 cm soil layer compared to the lower soil layers (Table S3). Wheat straw amendment increased the concentration of PCaCl2 in the WSNP compared to the NP root-affected soil at 33 DAF (Table S4).
Microbial response to dynamics of extractable C:N:P in soil
Time and straw treatment affected CO2 emission (F = 12.5, p < 0.001, Fig. 2C), interacting with soil depth (F = 2.5, p < 0.05; Table S3), but not with the factor soil compartment. Straw incorporation initially increased CO2 emissions (Fig. 2C). More CO2 was emitted from the 3–5 cm soil layer compared to the 20–30 cm soil layer at 20 and 40 DAF (Table S3).
Emission of N2O changed with time (F = 201.1, p < 0.001) and treatment (F = 6.1, p < 0.05, Table S2), with a slight interaction of treatment and soil depth (F = 3.2; p < 0.05). N2O emission was significantly lower in the WSNP compared to the NP treatment at 84 DAF. Occasionally, more N2O was emitted from the NP compared to the WSNP treatment also from greater soil depths (Table S3).
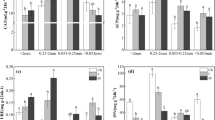
There was no obvious wheat straw treatment effect on the abundance of 16S rRNA genes in bulk and root-affected soil at 49 DAF (Fig. S3A), indicating comparable abundance of bacteria. However, in the root-affected soil of the WSNP treatment the abundance of 16S rRNA was significantly increased compared to the NP treatment at 49 DAF (Fig. 3A), which points to an increased activity of bacteria in the root-affected soil of the WSNP treatment. An initially increased abundance of nirK genes and nosZ transcripts in bulk soil (0 DAF) disappeared or reversed at 49 DAF, assuming no systematic bias on the experiment (Fig. 3F, S3E).
Wheat straw effects on (A) overall bacterial activity (16S rRNA transcripts), (B) ammonia-oxidizing activity of archaea (AOA), (C) ammonia-oxidizing activity of bacteria (AOB), (D) nitrite reductase activity (nirS), (E) nitrite reductase activity (nirK), and (F) nitrous oxide reductase activity (nosZ) in bulk and root-affected soil of the NP (control) and the WSNP (wheat straw) treatment zero days after amendment and fertilization (0 DAF) and 49 DAF (29 days after barley transplanting) in soil dry matter (dm). Significances between the treatments are illustrated with: ns (p > 0.05), * (p < 0.05), ** (p < 0.01), and *** (p < 0.001)
Compared to AOA, the abundance of amoA transcripts of AOB was lower in the WSNP bulk soil at 49 DAF (p = 0.057). This might point to a negative effect of WSNP on bacterial ammonia oxidizers, while no such shift occurred in soil of the NP treatment or in the WSNP root-affected soil. This was accompanied by soil stoichiometric shifts towards larger C:P and C:N ratios in the root-affected soil, but lower N:P ratios in the WSNP treatment at 49 DAF (Fig. 4A–C).
Box plot of wheat straw effects on stoichiometric nutrient ratio mean values of the NP (control) and the WSNP (wheat straw) treatment in bulk soil zero days after wheat straw amendment and fertilization (0 DAF) and in bulk and root-affected soil 49 DAF (29 days after barley transplanting) in the soil layer 3–5 cm (below the treatment application zone). (A) Molar C:P ratio (log scale) based on DOC and PCaCl2, (B) molar N:P ratio (log scale) based on Nmin (exchangeable NH4+ and NO3−) and PCaCl2, (C) molar C:N ratio (log scale) based on DOC and extractable Nmin, and (D) molar nitrous oxide (N2O) emission (log scale). Units are displayed at the y-axis. Arrows and asterisks mark significant shifts between the treatments in the according soil compartment at p ≤ 0.05. More details in Table S2
In root-affected soil of the WSNP treatment, the abundance of nirS and nirK transcripts, as well as of nosZ genes and transcripts, was larger compared to the NP treatment at 49 DAF (Fig. S3 and Fig. 3D–F), indicating a strong increase of denitrifiers capable of complete denitrification both in abundance and activity as a result of the WSNP addition. These shifts in the root-affected soil after wheat straw incorporation occurred simultaneously with shifts of the soil C:N:P stoichiometry at 49 DAF (Fig. 4A–C).
Cmic (F = 41.4, p < 0.001; Fig. 5A) and Nmic (F = 63.3; p < 0.001; Table S5) were affected by treatment and soil depth. Wheat straw mostly increased the Cmic by 124% in the first 5 cm of the WSNP compared to the NP soil at 90 DAF. Even at a soil depth of 10 to 30 cm, the WSNP treatment revealed an increased Cmic compared to the NP control (Table S5).
Wheat straw effects on plant parameters and microbial biomass mean values (± standard deviation) of the NP (control) and the WSNP (wheat straw) treatment. (A) Microbial biomass (µg C g−1) 90 days after wheat straw amendment and fertilization (DAF) in different soil depths from soil surface, and (B) number of tillers (counts), total biomass (g plant−1), shoot biomass (g plant−1), root biomass (g plant−1) 90 DAF. Asterisks indicate significant differences between the treatments at a certain incubation time at p ≤ 0.05 (Mann–Whitney U or t test). More details available in Tables S5 and S6
Plant responses to dynamics of extractable C:N:P in soil
The relative leaf greenness (SPAD value) was affected by the factor time (F = 6.6, p < 0.01), indicated by an increase in SPAD values of 39% and 20% in the NP and WSNP treatment between 51 and 90 DAF, respectively (Table S6). Besides the leaf number, tiller number was increased (by 1.8 tillers plant−1) in the WSNP compared to the NP treatment at 90 DAF (Fig. 5B). Root biomass per plant was increased by 125% in the WSNP compared to the NP treatment (Fig. 5B, Table S6). Roots of the WSNP treatment contained significantly less C (17%) compared to the NP treatment, lowering the root C:N ratio. Shoot C and N was not significantly different in the WSNP compared to the NP treatment (Table S6).
Discussion
Overall, we aimed to evaluate if a shallow wheat straw incorporation in combination with top-dressing mineral fertilization has a more positive effect on plant performance due to beneficial changes in microbial activity and nutrient stoichiometry than mineral fertilization alone. In order to allow a small-scale soil sampling with minimal effects on overall soil C and N dynamics, we developed special rhizotrons with resealable ports.
Improved plant performance after straw incorporation with mineral fertilization
Wheat straw addition significantly increased microbial biomass (Cmic and Nmic, Table S5, Fig. 5A), indicating microbial N immobilization, without a restricted nutrient availability to plants as frequently reported (e.g., Cheshire et al. 1999). In soils with low nutrient accessibility, organic C amendments can lead to a competitive microbial nutrient immobilization and “N-lock” effects on plant growth and yield (Ganry et al. 1978; Hauggaard-Nielsen et al. 1998; Elazab et al. 2016). However, incorporation of accessible organic C can also lead to microbial mining of N from soil organic matter (Fontaine et al. 2011), which could be beneficial for plant nutrition after decay of these microorganisms at later times (Reichel et al. 2018). In the present study, straw significantly reduced the availability of mineral N (NH4+ and NO3−) in soil (Tables S2–4), however, only to a level without signs of N deficiency of plants (chlorophyll content, Table S6). In a mesocosm experiment, van Duijnen et al. 2018 found no effect of wheat straw, but negative effects of sawdust addition on barley biomass and grain yield. Our results clearly showed that a shallow incorporation of wheat straw in spring with adequate mineral fertilization does not limit nutrient supply and growth of plants.
This study showed that a straw incorporation in spring keeps a substantial amount of nutrients bound in the microbial biomass in the upper soil layers, making them potentially more accessible to young plant roots if released in the right amount and at the right time. The nutrient retention effect was indicated by consistently increased microbial biomass (Fig. 5A), approximately 25% lower mineral N concentration, and 3% reduced NO3− leaching in the straw treatment (Fig. 2B). Our study also indicates that the wheat straw treatment-mediated N:P shifts in the bulk soil may temporarily lower the abundance of ammonia-oxidizing bacteria and related NO3− loss from soil (AOB, Fig. S3C). Lower abundances of AOB in bulk soil appear to be related to their low competitiveness for exchangeable NH4+ compared to other N-immobilizing soil microorganisms (Norman and Barrett 2014). Our data also indicate that the presence of roots might override this straw-mediated effect of N:P shifts on ammonia-oxidation in bulk soil (Fig. S3C). Nonetheless, evidence from the literature suggests that organic soil amendments such as wheat straw protect nutrients from leaching during wintertime through incorporation in microbial biomass (Van Duijnen et al. 2018; Liu et al. 2021), potentially making them more accessible for root uptake in the following year. Young plants with shallow root systems could especially benefit from this type of microbially mediated nutrient storage near the soil surface. In addition, the present study shows that young crop plants benefit from a shallow incorporation of wheat straw with mineral fertilization in spring, proving our first hypothesis.
Straw-mediated nutrient shifts and microbial activation in root-associated soil
Besides the possibility of a colonization of roots with beneficial microorganisms that outcompete pathogens (Iswandi et al. 1987; Schulz-Bohm et al. 2018; Tang et al. 2020), net positive effects on plant performance might also relate to an improved re-mineralization of previously immobilized nutrients.
Close interactions between wheat straw and barley roots (Fig. 4S) caused shifts towards a greater C:P and C:N ratios in the root-affected soil (Fig. 4A, C), which significantly enhanced microbial activity (16S rRNA transcripts, Fig. 3A). According to the plant-microbial interactions framework of Čapek et al. (2018), wheat straw may have optimized the difference between the nutrient limitation levels of plants (N:P) and soil microorganisms (C:N:P), resulting in more cooperative (beneficial) than competitive plant-soil microbial interactions. Lemanski et al. (2019) found additional evidence that reduced microbial C limitation during the growing season could improve crop yields. Literature shows that temporal and factorial N:P availability are expected to affect barley growth performance by altering root system responses and C investment in nutrient foraging (Kumar et al. 2020; van Duijnen et al. 2021).
Furthermore, surplus of C from living roots and residues leads to microbial activation, and as a result, microorganisms might start to decompose straw-derived soil organic matter to acquire nutrients (Kumar et al. 2016; Clocchiatti et al. 2021). Fontaine et al. (2004) also showed that increased supply of readily available C could accelerate mineralization of soil organic matter to fulfill microbial nutrient demand. Easy available C in the form of glucose, mimicking root C exudates, might regulate the nutrient release by microbial mining in soil organic matter (Shahbaz et al. 2018).
In addition, the significant increase in total microbial biomass C (Cmic, Fig. 5A) in contrast to bacterial 16S rRNA gene (Fig. S3A) might indicate the involvement of root-associated fungi. It is known that saprotrophic fungal biomass increases after the addition of cellulose-rich materials to the soil. Clocchiatti et al. (2021) showed increased biomass and activity of mycorrhizal fungi after the addition of C-dominated amendments such as sawdust. Wheat straw promoted fungal growth under N-unlimited soil conditions (Henriksen and Breland 1999), as in the present experiment, by creating nutrient patches that were attractive to root and fungal foraging (Cheng et al. 2016). Mycorrhizal fungi as potential plant symbionts are capable of enhancing decomposition of organic patches, potentially supporting the plant nutrient supply (Hodge et al. 2001). These fungi possess strong exoenzymes of which phosphatase activity in soil of the straw treatment could explain the increased P availability (Table S4; Joner and Jakobsen 1995; Akhtar et al. 2018). In the initial decomposition phase of wheat straw, a considerable proportion of the microbial biomass is likely related to microorganisms that are rapidly outcompeted and die after depletion of organic C (Reichel et al. 2018). This newly formed microbial necromass is suggested as nutrient reservoir for microorganisms and plants after remobilization by exoenzymes (Shabaz et al. 2017). We assume that C-rich amendments as applied in this study help to build up such organic nutrient reserves from which microorganisms and plants can later benefit (Fig. S4, Clocchiatti et al. 2021).
We could show that N cycling was enhanced by root activity in root-affected soil near the straw detritusphere, which appeared to be associated with greater denitrifying activity (Fig. 3D–F). Yang et al. (2019) suggest that wheat straw has the potential to stimulate soil N cycling in a manner beneficial to plant growth. In addition, our study suggests that root interaction with straw favors complete denitrification to N2 rather than N2O (Fig. 3D–F; Fig. 4D), which could be considered indicative of a more closed N cycle than in bulk soil. New primers for Clade I nosZ might even detect a higher number of responding taxa than those used in the present study (Table 1; Zhang et al. 2021a, b). Zhou et al. (2020) relate a more complete denitrification to straw-induced shifts in the C:N ratio, and it is well known that shifts in C:N:P ratio have a major impact on denitrification and N2O:N2 emission ratio (Smith and Tiedje 1979; Firestone et al. 1980; Paul and Beauchamp 1989; Mehnaz and Dijkstra 2016).
In accordance with our second hypothesis, the results of this study suggest that positive effects on plant performance are related to straw-mediated shifts of extractable C:N:P ratio, microbial activity, and N cycling in root-affected soil.
Conclusion
Under conditions as applied in this study, we could show that a shallow wheat straw application may improve the microbial regulation of top-dressing fertilizer nutrients with benefits on plant growth in spring. We could validate the hypothesis that shallow wheat straw incorporation with mineral nutrients has a more positive effect on plant performance than mineral fertilization alone. The in-house developed rhizotrons with resealable soil sampling ports made it possible to take samples at small scale along a 2D soil profile and during plant growth with minimal disturbance, which provided insight in the overall C and N cycle processes and plant-soil-microbial interactions. Therefore, we have been able to link plant performance to changes in extractable C:N:P, stimulated microbial activity, and increased N cycling in root-affected soil, thereby validating hypothesis two. Based on our results, we think that plants roots are able to optimize their nutrient supply by targeted root exudation of C as a prerequisite to stimulate re-mineralization of nutrients previously immobilized in straw-mediated microbial biomass. Further research has to evaluate if plant roots actively prime mining of previously immobilized nutrients in the straw detritusphere or if effects of pathogen suppression and growth promotion are dominating.
Data availability
All data are included in figures and tables. Raw data will be available from the BonaRes Repository https://datenzentrum.bonares.de/research-data.php.
Code availability
Not applicable.
Abbreviations
- NP:
-
Treatment with N and P fertilization
- WSNP:
-
Treatment with wheat straw incorporation and N and P fertilization
- DAF:
-
Days after wheat straw amendment and fertilization
- S:
-
Supplementary information
References
Akhtar K, Wang W, Ren G, Khan A, Feng Y, Yang G (2018) Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil till Res 182:94–102. https://doi.org/10.1016/j.still.2018.05.007
Amelung W, Welp G, Horn R (2018) Microbial and abiotic N transformations in soil. In: Amelung W, Blume H-P, Fleige H, Horn R, Kandeler E, Kögel-Knabner I, Kretzschmar R, Stahr K, Wilke B-M (eds) Scheffer/Schachtschabel Soil Science, 17th German edn. Springer Spektrum, Berlin, pp 491–581. https://doi.org/10.1007/978-3-662-55871-3_9
Bach HJ, Tomanova J, Schloter M, Munch JC (2002) Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. J Microbiol Methods 49:235–245. https://doi.org/10.1016/S0167-7012(01)00370-0
Braker G, Fesefeldt A, Witzel K-P (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64:3769–3775. https://doi.org/10.1128/aem.64.10.3769-3775.1998
Čapek P, Manzoni S, Kaštovská E et al (2018) A plant–microbe interaction framework explaining nutrient effects on primary production. Nat Ecol Evol 2:1588–1596. https://doi.org/10.1038/s41559-018-0662-8
Cheng L, Chen W, Adams TS, Wei X, Li L, McCormack ML, Deforest JL, Koide RT, Eissenstat DM (2016) Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 9:2815–2823. https://doi.org/10.1002/ecy.1514
Cheshire MV, Bedrock CN, Williams BL, Chapman SJ, Solntseva I, Thomsen I (1999) The immobilization of nitrogen by straw decomposing in soil. Eur J Soil Sci 50:329–341. https://doi.org/10.1046/j.1365-2389.1999.00238.x
Clocchiatti A, Hannula SE, Hundscheid MP, KlienGunnewiek PJ, de Boer W (2021) Stimulated saprotrophic fungi in arable soil extend their activities to the rhizosphere and root microbiomes of crop seedlings. Environ Microbiol 23:6056–6073. https://doi.org/10.1111/1462-2920.15563
Dessureault-Rompré J, Nowack B, Schulin R, Luster J (2006) Modified micro suction cup/rhizobox approach for the in-situ detection of organic acids in rhizosphere soil solution. Plant Soil 286:99–107. https://doi.org/10.1007/s11104-006-9029-z
Elazab A, Serret MD, Araus JL (2016) Interactive effect of water and nitrogen regimes on plant growth, root traits and water status of old and modern durum wheat genotypes. Planta 244:125–144. https://doi.org/10.1007/s00425-016-2500-z
Fierer N, Schimel JP (2002) Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787. https://doi.org/10.1016/S0038-0717(02)00007-X
Firestone MK, Firestone RB, JM T (1980) Nitrous oxide from soil denitrification: factors controlling its biological production. Science 208:749–751. https://doi.org/10.1126/science.208.4445.749
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol lett 7:314–320. https://doi.org/10.1111/j.1461-0248.2004.00579.x
Fontaine S, Hénault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. https://doi.org/10.1016/j.soilbio.2010.09.017
Ganry F, Guiraud G, Dommergues Y (1978) Effect of straw incorporation on the yield and nitrogen balance in the sandy soil-pearl millet cropping system of Senegal. Plant Soil 50:647–662. https://doi.org/10.1007/BF02107216
Griffiths BS, Spilles A, Bonkowski M (2012) C:N: P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol Process 1:6. https://doi.org/10.1186/2192-1709-1-6
Hauggaard-Nielsen H, de Neergaard A, Jensen LS, Høgh-Jensen H, Magid J (1998) A field study of nitrogen dynamics and spring barley growth as affected by the quality of incorporated residues from white clover and ryegrass. Plant Soil 203:91–101. https://doi.org/10.1023/A:1004350215467
Henriksen TM, Breland TA (1999) Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol Biochem 31:1121–1134. https://doi.org/10.1016/S0038-0717(99)00030-9
Henry S, Baudoin E, López-Gutiérrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Methods 59:327–335. https://doi.org/10.1016/j.mimet.2004.07.002
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189. https://doi.org/10.1128/aem.00231-06
Hilton RJ, Bhar DS, Mason GF (1969) A rhizotron for in situ root growth studies. Can J Plant Sci 49:101–104. https://doi.org/10.4141/cjps69-018
Hodge A, Campbell C, Fitter A (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299. https://doi.org/10.1038/35095041
Iswandi A, Bossier P, Vandenabeele J, Verstraete W (1987) Effect of seed inoculation with the rhizopseudomonad strain 7NSK2 on the root microbiota of maize (Zea mays) and barley (Hordeum vulgare). Biol Fertil Soils 3:153–158. https://doi.org/10.1007/BF00255776
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass. Calibration of the kEC value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Joner EJ, Jakobsen I (1995) Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol Biochem 27:1153–1159. https://doi.org/10.1016/0038-0717(95)00047-I
Kimber RWL (1973) Phytotoxicity from plant residues. Plant Soil 38:543–555. https://doi.org/10.1007/BF00010694
Kuchenbuch R, Jungk A (1982) A method for determining concentration profiles at the soil-root interface by thin slicing rhizospheric soil. Plant Soil 68:391–394. https://doi.org/10.1007/BF02197944
Kumar A, Kuzyakov Y, Pausch J (2016) Maize rhizosphere priming: field estimates using 13C natural abundance. Plant Soil 409:87–97. https://doi.org/10.1007/s11104-016-2958-2
Kumar A, van Duijnen R, Delory BM, Reichel R, Brueggemann N, Temperton VM (2020) Barley shoot biomass responds strongly to N: P stoichiometry and intraspecific competition, whereas roots only alter their foraging. Plant Soil 453:515–528. https://doi.org/10.1007/s11104-020-04626-w
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. https://doi.org/10.1038/nature04983
Lemanski K, Armbruster M, Bonkowski M (2019) Linking soil microbial nutrient limitation to fertilizer regime and sugar beet yield. Plant Soil 441:253–259. https://doi.org/10.1007/s11104-019-04114-w
Li Z, Reichel R, Xu Z, Vereecken H, Brüggemann N (2021) Return of crop residues to arable land stimulates N2O emission but mitigates NO3− leaching: a meta-analysis. Agron Sustain Dev 41:66. https://doi.org/10.1007/s13593-021-00715-x
Liu E, Yan C, Mei X, He W, Bing SH, Ding L, Liu Q, Liu S, Fan T (2010) Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 158:173–180. https://doi.org/10.1016/j.geoderma.2010.04.029
Liu B, Wang X, Ma L, Chadwick D, Chen X (2021) Combined applications of organic and synthetic nitrogen fertilizers for improving crop yield and reducing reactive nitrogen losses from China’s vegetable systems: A meta-analysis. Environ Pollut 269:116143. https://doi.org/10.1016/j.envpol.2020.116143
Lobet G, Draye X (2013) Novel scanning procedure enabling the vectorization of entire rhizotron-grown root systems. Plant Methods 9:1. https://doi.org/10.1186/1746-4811-9-1
Malhi SS, Nyborg M, Solberg ED, Dyck MF, Puurveen D (2011) Improving crop yield and N uptake with long-term straw retention in two contrasting soil types. Field Crops Res 124:378–391. https://doi.org/10.1016/j.fcr.2011.07.009
Mehnaz KR, Dijkstra FA (2016) Denitrification and associated N2O emissions are limited by phosphorus availability in a grassland soil. Geoderma 284:34–41. https://doi.org/10.1016/j.geoderma.2016.08.011
Michotey V, Méjean V, Bonin P (2000) Comparison of methods for quantification of cytochrome cd1-denitrifying bacteria in environmental marine samples. Appl Environ Microbiol 66:1564–1571. https://doi.org/10.1128/aem.66.4.1564-1571.2000
Nagel KA, Putz A, Gilmer F, Heinz K, Fischbach A, Pfeifer J, Faget M, Blossfeld S, Ernst M, Dimaki C, Kastenholz B, Kleinert A-K, Galinski A, Scharr H, Fiorani F, Schurr U (2012) GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct Plant Biol 39:891–904. https://doi.org/10.1071/FP12023
Nakano H, Morita S, Shigemori H, Hasegawa K (2006) Plant growth inhibitory compounds from aqueous leachate of wheat straw. Plant Growth Regul 48:215–219. https://doi.org/10.1007/s10725-006-0006-6
Neumann G, George TS, Plassard C (2009) Strategies and methods for studying the rhizosphere—the plant science toolbox. Plant Soil 321:431–456. https://doi.org/10.1007/s11104-009-9953-9
Nischwitz V, Mogwasi R, Zor S, Getenga Z, Kariuki DK, Günther K (2017) First comprehensive study on total contents and hot water extractable fraction of selected elements in 19 medicinal plants from various locations in Nyamira County, Kenya. Trace Elem Med Biol 39:54–61. https://doi.org/10.1016/j.jtemb.2016.08.001
Norman JS, Barrett JE (2014) Substrate and nutrient limitation of ammonia-oxidizing bacteria and archaea in temperate forest soil. Soil Biol Biochem 69:141–146. https://doi.org/10.1016/j.soilbio.2013.11.003
Paul JW, Beauchamp EG (1989) Denitrification and fermentation in plant-residue-amended soil. Biol Fertil Soils 7:303–309. https://doi.org/10.1007/BF00257824
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361. https://doi.org/10.1007/s11104-008-9568-6
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Retrieved from https://www.r-project.org/. Accessed 07.02.2021
Reichel R, Wei J, Islam MS, Schmid C, Wissel H, Schröder P, Schloter M, Brüggemann N (2018) Potential of wheat straw, spruce sawdust, and lignin as high organic carbon soil amendments to improve agricultural nitrogen retention capacity: an incubation study. Front Plant Sci 9:900. https://doi.org/10.3389/fpls.2018.00900
Rodgers-Gray BS, Shaw MW (2000) Substantial reductions in winter wheat diseases caused by addition of straw but not manure to soil. Plant Pathol 49:590–599. https://doi.org/10.1046/j.1365-3059.2000.00497.x
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712. https://doi.org/10.1128/AEM.63.12.4704-4712.1997
Schulz-Bohm K, Gerards S, Hundscheid M, Melenhorst J, de Boer W, Garbeva P (2018) Calling from distance: attraction of soil bacteria by plant root volatiles. ISME J 12:1252–1262. https://doi.org/10.1038/s41396-017-0035-3
Shahbaz M, Kuzyakov Y, Sanaullah M, Heitkamp F, Zelenev V, Kumar A, Blagodatskaya E (2017) Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils 53:287–301. https://doi.org/10.1007/s00374-016-1174-9
Shahbaz M, Kumar A, Kuzyakov Y, Börjesson G, Blagodatskaya E (2018) Priming effects induced by glucose and decaying plant residues on SOM decomposition: a three-source 13C/14C partitioning study. Soil Biol Biochem 121:138–146. https://doi.org/10.1016/j.soilbio.2018.03.004
Smith MS, Tiedje JM (1979) The effect of roots on soil denitrification. Soil Sci Soc Am J 43:951–955. https://doi.org/10.2136/sssaj1979.03615995004300050027x
Steinmann T, Welp G, Wolf A, Holbeck B, Große-Rüschkamp T, Amelung W (2016) Repeated monitoring of organic carbon stocks after eight years reveals carbon losses from intensively managed agricultural soils in Western Germany. J Plant Nutr Soil Sci 179:355–366. https://doi.org/10.1002/jpln.201500503
Süß A, Danner M, Obster C, Locherer M, Hank T, Richter K, Consortium E (2015) Measuring leaf chlorophyll content with the Konica Minolta SPAD-502Plus—theory, measurement, problems, interpretation. EnMAP Field Guides Technical Report, GFZ Data Services. OI: https://doi.org/10.2312/enmap.2015.010
Tang L, Xia Y, Fan C, Kou J, Wu F, Li W, Pan K (2020) Control of Fusarium wilt by wheat straw is associated with microbial network changes in watermelon rhizosphere. Sci Rep 10:12736. https://doi.org/10.1038/s41598-020-69623-6
Throbäck IN, Enwall K, Jarvis Å, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417. https://doi.org/10.1016/j.femsec.2004.04.011
Töwe S, Wallisch S, Bannert A, Fischer D, Hai B, Haesler F, Kleineidam K, Schloter M (2011) Improved protocol for the simultaneous extraction and column-based separation of DNA and RNA from different soils. J Microbiol Methods 84:406–412. https://doi.org/10.1016/j.mimet.2010.12.028
van Duijnen R, Roy J, Härdtle W, Temperton VM (2018) Precrop functional group identity affects yield of winter barley but less so high carbon amendments in a mesocosm experiment. Front Plant Sci 9:912. https://doi.org/10.3389/fpls.2018.00912
van Duijnen R, Uther H, Härdtle W, Temperton VM, Kumar A (2021) Timing matters: distinct effects of nitrogen and phosphorus fertilizer application timing on root system architecture responses. J Plant Interact 2:1–12. https://doi.org/10.1002/pei3.10057
VDLUFA (1991) Determination of the gravimetric water content (A 2.1.1), soil pH (A 5.1.1), and mineral N in soil profiles (A 6.1.4.1). In: Association of German Agricultural Analytic and Research Institutes, Method Book Volume 1, 4th German edn. VDLUFA-Verlag, Darmstadt
Wei J, Reichel R, Islam MS, Wissel H, Amelung W, Brüggemann N (2020) Chemical composition of high organic carbon soil amendments affects fertilizer-derived N2O emission and nitrogen immobilization in an oxic sandy loam. Front Environ Scihttps://doi.org/10.3389/fenvs.2020.00015
Yang H, Ma J, Rong Z, Zeng D, Wang Y, Hu S, Ye W, Zheng X (2019) Wheat straw return influences nitrogen-cycling and pathogen associated soil microbiota in a wheat–soybean rotation system. Front Microbiol 10:1811. https://doi.org/10.3389/fmicb.2019.01811
Youssef RA, Chino M (1987) Studies on the behavior of nutrients in the rhizosphere I: establishment of a new rhizobox system to study nutrient status in the rhizosphere. J Plant Nutr 10:1185–1195. https://doi.org/10.1080/01904168709363646
Zhang B, Penton CR, Yu Z, Xue C, Chen Q, Chen Z, Yan C, Zhang Q, Zhao M, Quensen JF, Tiedje JM (2021a) A new primer set for Clade I nosZ that recovers genes from a broader range of taxa. Biol Fertil Soils 57:523–531. https://doi.org/10.1007/s00374-021-01544-6
Zhang C, Lin Z, Que Y, Fallah N, Tayyab M, Li S, Luo J, Zhang Z, Abubakar AY, Zhang H (2021b) Straw retention efficiently improves fungal communities and functions in the fallow ecosystem. BMC Microbiol 21:52. https://doi.org/10.1186/s12866-021-02115-3
Zhao ZB, He JZ, Geisen S, Han LL, Wang JT, Shen JP, Wei WX, Fang YT, Li PP, Zhang LM (2019) Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 7:33. https://doi.org/10.1186/s40168-019-0647-0
Zhong Y, Liu J, Jia X, Shangguan Z, Wang R, Yan W (2020) Microbial community assembly and metabolic function during wheat straw decomposition under different nitrogen fertilization treatments. Biol Fertil Soils 56:697–710. https://doi.org/10.1007/s00374-020-01438-z
Zhou W, Jones DL, Hu R, Clark IM, Chadwick DR (2020) Crop residue carbon-to-nitrogen ratio regulates denitrifier N2O production post flooding. Biol Fertil Soils 56:825–838. https://doi.org/10.1007/s00374-020-01462-z
Acknowledgements
We thank the team of the workshop IBG-2-3-TAK of Forschungszentrum Jülich for manufacturing the rhizotrons, especially Ayhan Egmen, who translated the original rhizotron concept of Rüdiger Reichel and Nicolas Brüggemann into technical drawings, as well as Klaus Jürgen Reichel (former inventor at the Fraunhofer Gesellschaft) who invented the custom-made cutting tool, and supported the technical drawings of the sampling procedure. Particular thanks goes also to the ZEA-3 and IBG-3 laboratory staff (Franz Leistner and Holger Wissel) for supporting the greenhouse gas and chemical analyses. We thank the Quarzwerke Frechen GmbH, Buir-Bliesheimer Agrargenossenschaft eG, Agrarlager Eschweiler, RWZ Rhein-Main eG, and YARA GmbH and Co. KG (all in Germany) for the free supply of sand, seeds, and fertilizer. We also thank all involved INPLAMINT partners as part of the BonaRes initiative, and Roland Baatz (IBG-3) for the internal review of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The “INPLAMINT” project—Increasing agricultural nutrient-use efficiency by optimizing plant-soil-microorganism interactions—was supported by the BonaRes soil sustainability program of the Federal German Ministry for Education and Research (BMBF). Grant numbers: FKZ 031B0508A, 031B0508C, 031B0508H.
bundesministerium für bildung und forschung,FKZ 031B0508A,Nicolas Brüggemann,FKZ 031B0508C,Michael Schloter,031B0508H,Vicky M. Temperton
Author information
Authors and Affiliations
Contributions
Rüdiger Reichel and Nicolas Brüggemann designed the rhizoboxes and the experiment. Rüdiger Reichel prepared the material, collected, and analyzed the data. Zhijie Li supported the experiment and sampling. Nitrification and denitrification genes were analyzed and interpreted by Catherine Wambui Kamau, Viviane Radl, and Michael Schloter. Amit Kumar and Vicky Temperton supported the planning of the experiment and plant nutrient analyses. The first draft of the manuscript was written by Rüdiger Reichel, and all authors revised and commented on previous versions of the manuscript. All approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reichel, R., Kamau, C.W., Kumar, A. et al. Spring barley performance benefits from simultaneous shallow straw incorporation and top dressing as revealed by rhizotrons with resealable sampling ports. Biol Fertil Soils 58, 375–388 (2022). https://doi.org/10.1007/s00374-022-01624-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01624-1