Abstract
Global food wastage equates to about 1.3 billion tons per year, which causes serious environmental impacts. The objective of this study was to evaluate the influences of addition of digestate from food waste in comparison to a synthetic liquid urea ammonium nitrate solution on plant growth, rhizosphere bacterial community composition and diversity, and hyphal abundance of arbuscular mycorrhizal (AM) fungi. Plant and soil samples were collected at 25, 50, and 75 days after seedling emergence. Annual ryegrass growth was significantly increased by both liquid urea ammonium nitrate and digestate, and digestate was just as effective as liquid urea ammonium nitrate. Additionally, digestate (50 kg N ha−1) significantly increased AM fungal hyphae density. Liquid urea ammonium nitrate (50 kg N ha−1) significantly decreased AM fungal hyphae density compared with liquid urea ammonium nitrate (25 kg N ha−1) at DAE 75. Digestate and liquid urea ammonium nitrate applications significantly shifted the bacterial community composition and OTU richness and changed the abundance of microbial C and N-cycling genes, while application rates had no significant effect. Structural equation modeling showed that digestate and UAN addition both directly and indirectly affected bacterial, C and N cycling genes community composition; the indirect effects were related to increased soil NO3− content and reduced pH. This study showed that the use of digestate as a soil amendment can be environmentally effective and can provide a sustainable supply of nutrients that increases soil organic C. Moreover, the use of digestate can readily be incorporated into agricultural practices with potentially less impact on soil microflora diversity and function than conventional fertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large quantities of food waste resulting from unused consumable food items or rejected from produce from manufacturers can have negative impacts on the environment if they are not managed adequately (Buhlmann et al. 2019). Currently, global food wastes are estimated at 1.3 billion tons per year and are expected to increase by 53% by 2025 (Adhikari et al. 2006; FAO 2011). About 97% of global food wastes ends up in landfill, where it readily decomposes to create various problems such as odor, leachate pollution of shallow and deep waters, and methane emissions (Melikoglu et al. 2013). Food waste management aims to encourage the recycling of organic waste by soil application of the waste to soil as an amendment (Tampio et al. 2016). Anaerobic digestion of various organic wastes or food for renewable energy and the production of nutrient-rich liquid and/or solid digestates are established measures that can alleviate loads on landfill while simultaneously recovering nutrients from waste resources (Zarezadeh et al. 2019).
The use of chemical fertilizers in agroecosystems can influence soil microbial communities and cause a decline in soil organic matter content (Kibblewhite et al. 2008; Liu and Greaver 2010). Specifically, the addition of N fertilizer can alter the metabolic activities of microbial communities that decompose soil C pools (Ramirez et al. 2012). For instance, Fierer et al. (2007) observed that shifts in the specific community were responsible for the decrease in decomposition rate and that an N addition decreased the relative abundance of oligotrophs that are adept at catabolizing recalcitrant C. In addition, N fertilizer addition can increase microbial N-cycling potential (Zhang et al. 2019). Furthermore, N addition can also inhibit many fungal activities through shifts in nutrient availability (Li et al. 2014a; Phillips et al. 2019), including those of arbuscular mycorrhizal (AM) fungi, which form symbiotic associations with many vascular plants and play a significant role in mediating the flow of mineral nutrients from the soil to the host plant (Phillips et al. 2019). A previous study showed that AM fungal extraradical hyphae can assimilate N mainly in the form of NH4+ and possibly NO3− and the hyphal abundance of AM fungi can be decreased under conditions of NH4+ supply (Zheng et al. 2014).
Utilization of digestate derived from food waste presents some advantages compared with synthetic N applications due to its plant-available nutrient content, including all of the necessary macro- and micronutrients. Moreover, digestate contains other organic elements, including some plant hormones and/or other substances not easily identifiable, which can also result in positive influences on plant growth and development (Möller et al. 2008). Application of digestate leads to higher amounts of organic C compounds and N (present as NH4+), which can play an important role in potentially increasing the soil C balance (Nielsen et al. 2011) and crop yield (Alburquerque et al. 2012b). In this context, the recovery of nutrients from food waste-derived digestates in agricultural systems has an important role by reducing the use of mineral fertilizers, and this leads to positive effects with respect to resource conservation and soil quality maintenance (García-Sánchez et al. 2015). Therefore, the use of digestate is an important component of integrated nutrient management for sustainable agriculture (Tampio et al. 2016). Recent findings reported that anaerobic digestates positively affect forage crop yields, especially grasses, with increases in plant growth either similar or exceeding those from equivalent amounts of traditional mineral fertilizers (Coelho et al. 2019). On the other hand, the utilization of digestate as an N source in agriculture can reduce costs (Sigurnjak et al. 2017) and environmental impacts (Möller 2015).
Soil microorganisms are central to soil ecological functioning, by providing several ecosystem services (Zeng et al. 2016). Microbial communities play a key role in organic matter transformation and in geochemical cycling, and strongly influence the soil physical characteristics as well as plant growth (Dennis et al. 2010). Therefore, soil microbial function and activity can reflect indicators of soil quality (Andrews et al. 2004; Gianfreda and Ruggiero 2006; Stott et al. 2010; Giacometti et al. 2013; Schloter et al. 2018); maintenance of soil functionality and diversity is essential for sustainable agricultural production. Indeed, some previous studies showed that digestate application to soil provided a source of available nutrients and had a positive impact on soil microbial community composition and diversity (García-Sánchez et al. 2015), microbial respiration, and enzyme activities (Gielnik et al. 2019). Elsewhere, only minor or non-significant soil microbial changes have been reported (Andruschkewitsch et al. 2013). In another study, application of anaerobic digestate stimulated soil bacterial growth, but not fungal growth (Walsh et al. 2012a). Food waste can contain substantial proportions of nitrogenous material which can produce high concentrations of ammonia as the waste is digested (Dai et al. 2017). Ammonia is necessary for microbial growth in anaerobic digestates, but excessive ammonia concentrations can lead to inhibition of microbial activity (Buhlmann et al. 2019).
A number of soil microbial changes have been related to functional genes associated with N cycling (Sapp et al. 2015; Zhang et al. 2019) and C transformations (Kibblewhite et al. 2008). The rhizosphere microbial community may stimulate or inhibit N and C cycling thereby influencing soil organic matter and nutrient availability to the plant (Zhu et al. 2014). The potential function of the microbial community can be estimated by quantifying the abundance of functional genes related to C and N cycling and assessing how they are impacted by agricultural management practices (Manoharan et al. 2017; Mickan et al. 2018, 2019). However, consistent predictions of how the soil microbial community responds to digestate application are difficult due to the inherent heterogeneity of both soil and digestate across studies. Therefore, consideration of a broader understanding of the dominant features of similar soil microbes and ecological theories may be useful in predicting changes in community composition following amendment (Mickan et al. 2018, 2019). For example, at the class or phylum level, gram-negative bacteria are often described as r-strategist and characterized as fast-growing with low substrate affinity (DeVries and Shade 2013). Application of digestate can decrease the relative abundance of gram-negative soil bacteria (Planctomycetes and Bacteroidetes), while the relative abundance of gram-positive (Firmicutes) rhizosphere bacteria exhibited the opposite trend (Caracciolo et al. 2015; Sapp et al. 2015). Following digestate application to nutrient-limited soil, slowly growing microorganisms (K-strategists) became dominant (Sapp et al. 2015). Examining ecological strategies of soil bacteria based on r-/K-selection theory can be a useful framework for predicting functional changes associated with soil bacteria following digestate application (DeVries and Shade 2013).
To date, although the effects of amendment with digestate on soil microbial characteristics have been reported, these studies have focused on digestate from manure (Alburquerque et al. 2012b; Caracciolo et al. 2015; Nõlvak et al. 2016; Sigurnjak et al. 2017). The effects of different types of digestate application on soil microbial communities vary (Coelho et al. 2019). Furthermore, these studies tested only a single time point and were therefore unable to shed light on temporal dynamics of rhizosphere communities associated with digestate amendment. On the other hand, less is known about how digestate affects soil C- and N-cycling potentials, including temporal dynamics. Therefore, in our study, we evaluated how plant growth, rhizosphere bacterial community composition and function, and hyphal abundance of AM fungi were affected by digestate from food waste and liquid urea ammonium nitrate (UAN) over time in a short-term pot experiment. The aim was to provide information relevant to the use of digestate from the anaerobic processing of food waste as a substitute for mineral fertilizers (especially N-fertilizer) in sandy semiarid agricultural soil. The hypotheses were (i) that digestate would significantly influence the soil microbial community composition and function, as well as plant growth over time in comparison to liquid urea ammonium nitrate; (ii) that digestate application would increase the relative abundance of Firmicutes and Actinobacteria and enhance the potential activity of enzymes associated with C degradation and N transformations. Conversely, liquid urea ammonium nitrate application was expected to reduce the rhizosphere bacterial potential function; and (iii) that application of digestate and liquid urea ammonium nitrate together would decrease the AM fungal colonization and alter the bacterial community composition.
Materials and methods
Experimental design
A glasshouse pot experiment was established using annual ryegrass (Lolium rigidum), which is an important component of pastures in Australia. The experimental design consisted of five soil treatments with four replicates using a randomized block design. The soil treatments included two application rates (25 or 50 kg N ha−1) of either digestate or liquid urea ammonium nitrate and an unamended (CK).
Soil collection, digestate, and liquid urea ammonium nitrate
The experiment was established using plastic pots (dimeter 14.0 cm, depth 13 cm) filled with 1.6 kg of soil. The soil was collected from the top 20 cm from an agricultural farm at Katanning in south-western Australia (33°45′S, 117°27′E). Soil was air-dried, sieved (2 mm), and mixed before use. The soil properties were exchangeable NH4+-N 9.00 mg kg−1, NO3−-N 3.00 mg kg−1, P 22.0 mg kg−1, K 42.0 mg kg−1, S 35.1 mg kg−1, organic C 1.77%, conductivity 0.37 dS/m, and pH 5.5. The soil is a yellow sandy duplex or Typic Palexerult (USDA Soil Taxonomy). The digestate was obtained from a mesophilic anaerobic digestion facility near Perth in Western Australia, primarily treating food waste. Specifically, a 2500-m3 digester that treated mixed wastes at a hydraulic retention time of 30 days at 35 °C was used (Buhlmann et al. 2019). A detailed analysis of the nutrient composition of the digestate is shown in Table 1. The ratios of digestate application were 5 g and 10 g (fresh digestate) per 100 g dry soil (equivalent to a field application of 25 and 50 kg N ha−1, respectively). This application dose was selected to avoid low inputs of organic C to soil and for keeping the N addition at an optimal rate. Fertilizer N was applied as 32% liquid urea ammonium nitrate broadcasted on the soil surface and mixed with topsoil. The liquid urea ammonium nitrate application rates were of 0.05 g and 0.1 g per 100 g soil (equivalent to a field application of 25 and 50 kg N ha−1, respectively). Digestate and liquid urea ammonium nitrate were added manually and mixed thoroughly with the soil. No other fertilizers were added during the experiment period. After an equilibration phase of 10 days in the glasshouse, the plants were sown.
Seed germination and planting
Ryegrass seeds were soaked with deionized water in Petri dish until the free radicle emerged. Germinated seeds were planted at 10 mm depth in each pot, arranged in ten pairs of equal spacing. Plants were thinned to five per pot after emergence. Pots were maintained under glasshouse conditions and watered accordingly to maintain soil moisture at about 80% field capacity in the cause of the experiment. All pots were weighed daily to determine the soil water content. To evaluate the temporal dynamics of the rhizosphere microbiome, three destructive harvests for each treatment were performed at 25, 50, and 75 days after seedling emergence (DAE), representing various stages of plant growth (seedling, tillering, peak tillering). Shoot and root biomass were measured at each harvest after oven-drying at 60 °C for 72 h.
Soil analysis
At each harvest, roots were carefully removed from the bulk soil and gently shaken to remove loosely adhering soil. The more tightly adhering rhizosphere soil (Mickan et al. 2018) was collected and stored at 4 °C for soil measurements and − 20 °C for genomic DNA extraction.
The electrical conductivity (EC) and pH were measured using a probe inserted into water mixtures (1:5 soil/water ratio). Total soil C (TC) and N (TN) were measured using an Elementar Analyser (Vario Macro CNS, Elementar, Germany). Dissolved organic C was extracted using a combination of 20 g soil to 80 mL 0.5 M K2SO4 and analyzed using an OI Analytical Aurora 1030 Wet Oxidation TOC Analyzer (College Station, TX, USA). The same mixture (20 g with 80 mL 0.5 M K2SO4) was used for measuring the soil N-nitrate (NO3−-N) and the soil exchangeable ammonium-N (NH4+-N), the content of exchangeable NH4+ was measured using the salicylate–nitroprusside method (Searle 1984) and NO3− concentration using the hydrazine reduction method (Kempers and Luft 1988) on an automated flow injection Skalar AutoAnalyser (San plus, Skalar Analytical, The Netherlands).
AM fungal root colonization and extraradical hyphal length assessment
At each harvest, roots were carefully washed free of soil, and a subsample of known weight (0.5 g fresh weight) was taken from each root sample. Root sub-samples were cut into 1 cm pieces and cleared in 10% KOH, acidified, and stained with Trypan blue (0.05%) in lactoglycerol (1:1:1 lactic acid/glycerol/water) for AM fungal quantification (Abbott and Robson 1981). The percentage of AM fungal structures was determined microscopically at 200× magnification with the gridline intersect method (Giovannetti and Mosse 1980). Soil cores (1-cm diameter) were taken from the center of the pots to a depth of 7 cm. All soil cores were stored at 5 °C for hyphal extraction. Hyphal length in the soil was measured as described by Jakobsen et al. (1992).
DNA extraction, PCR amplification, and sequencing
DNA was extracted from 0.4 g of rhizosphere soil using a Power Soil® DNA Isolation Kit (Mo Bio, Carlsbad, CA, USA) and following the protocol of the manufacturer. Extracted DNA was quantified (Qubit, Life Technologies, Australia) and adjusted to 1 ng/μL using molecular-grade water and stored at − 20 °C until further analysis. The DNA preparation and sequencing library preparation were performed following the recommendations described by Scholer et al. (2017) and Vestergaard et al. (2017). Amplification of the target 16S rRNA genes was carried out following the protocol of Mickan et al. (2018) using 27F/519R bacterial primers (Caporaso et al. 2010) amended with the barcodes of Golay (Caporaso et al. 2012) with negative controls.
Bioinformatics and PICRUSt
DNA sequencing was on the Illumina Mi-seq platform. Paired-end reads were assembled by aligning the forward and reverse reads using PEAR (version 0.9.5) (Zhang et al. 2014). The primers were identified, and using Quantitative Insights into Microbial Ecology (QIIME 1.8) (Caporaso et al. 2010) USEARCH (version 8.0.1623, Edgar et al. 2011) and UPARSE software, the trimmed sequences were processed. Using the USEARCH, sequences were denoized, quality filtered, and chimera checked according to abundance. The reads were mapped back to the operational taxonomic units (OTUs) based on 97% identity to obtain the number of reads in each OTU. Using the Greengenes database5 to assign the QIIME taxonomy (version 13_8, Aug 2013), a Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (https://picrust.github.com) was performed (Langille et al. 2013). The genes characterized were those identified in C and N cycling (Mickan et al. 2018). The metagenomes were collapsed into the Kyoto Encyclopedia of Genes and Genomes. For unknown reasons, MiSeq sequencing of one of the samples belonging to digestate (25 kg N ha−1) at the second sampling and one of the samples belonging to digestate (50 kg N ha−1) and one of the samples belonging to liquid urea ammonium nitrate (50 kg N ha−1) at the third sampling failed. Thus, we removed these three samples in the analysis.
Statistical analysis
One-way analysis of variance (ANOVA) was used to evaluate the effect of digestate and liquid urea ammonium nitrate application on plant growth, mycorrhizal parameters, soil parameters, bacterial community composition, and relative abundance, with significant results further analyzed with post hoc test for multiple comparisons. Bray–Curtis dissimilarity was used to analyze bacterial community compositional changes at OTU level, and the first axis on non-metric multidimensional scaling (NMDS1) was used in subsequent structural equation modeling (SEM) analysis. The significance of different fertilizer driving bacteria community composition was assessed with permutational multivariate analysis of variance (PERMANOVA) using distance matrices (adonis function) and square root-transformed OTU relative abundance data. All data analyses were conducted in the R statistical environment. Structural equation modeling was carried out to test for directly and indirectly relationships among observed factors. In our study, SEM analysis was used to gain an understanding of how digestate and urea application mediate alterations in AM fungal colonization and bacterial diversity and composition, and then affects the plant biomass. In SEM analysis, the data were fitted to the models using the maximum likelihood estimation method. Each variable has a relative contribution degree shown in arrow in path diagram. Path coefficients and explained variability are estimated in models. Adequate model fits were indicated by the χ2 test (df > 5; P > 0.05) and a low RMSEA (P < 0.05).
Results
Changes in soil properties after fertilization
The soil physicochemical properties at three harvest times are shown in Table 2. Soil pH markedly decreased with the increase in fertilizer application rate. Inorganic N (exchangeable NH4+ and NO3−) and dissolved organic C were correlated with the sampling period (Table 2). Both dissolved organic C and exchangeable NH4+-N content increased over time. The exchangeable NH4+-N content was higher at DAE 75 and ranged from 27.6 to 32.7 mg kg−1 and DOC ranged from 34.9 to 53.6 g kg−1, across the treatments. Exchangeable NH4+-N was significantly higher in liquid urea ammonium nitrate treatments at DAE 75 than in the digestate and control treatments, while there were no significant differences between fertilization rates. DOC in digestate (50 kg N ha−1) was 6.26% and 29.1% higher at DAE 25 and DAE 75, respectively, compared with digestate (25 kg N ha−1). However, DOC for liquid urea ammonium nitrate (50 kg N ha−1) was 10.2% and 27.6% lower at DAE 50 and DAE 75 compared with liquid urea ammonium nitrate (25 kg N ha−1). In addition, NO3−-N content was higher at DAE 25, decreasing over time. Neither digestate nor liquid urea ammonium nitrate application significantly impacted soil total organic C and N.
Plant growth, AM fungal colonization, and extraradical hyphal length
Dry shoot biomass significantly varied with harvest time (Fig. 1a). Based on an average of the two amendment rates, shoot biomass in digestate and liquid urea ammonium nitrate were significantly increased by 51% and 42% at DAE 25, 68% and 69% at DAE 50, and 74% and 75% at DAE 75, respectively (Fig. 1a). Shoot biomass in the higher rates of both digestate (50 kg N ha−1) and liquid urea ammonium nitrate (50 kg N ha−1) was 28% and 23% more than those of their respective lower rates (25 kg N ha−1) at DAE 50, and 44% and 38% more at DAE 75 (Fig. 1a). Similarly, root biomass varied with harvest time (Fig. 1b). Across the two amendment rates, root biomass in digestate and liquid urea ammonium nitrate increased by 55% and 58% at DAE 50 and by 57% and 61% at DAE 75, respectively (Fig. 1b). Root biomass in the higher rates of both digestate (50 kg N ha−1) and liquid urea ammonium nitrate (50 kg N ha−1) were 18% and 18% more than those of their respective lower rates (25 kg N ha−1) at DAE 75.
Total shoot dry mass (a), root dry mass (b), AM fungal colonization (c), and hyphal length in soil (d) of annual ryegrass assessed at 25, 50, and 75 days after emergence (DAE). DAE 25, DAE 50, DAE 75 refer to 25, 50, and 75 days of annual ryegrass after emergence. CK: control; Diges_25: digestate, 25 kg N ha−1; Diges_50: digestate, 50 kg N ha−1; UAN_25: liquid urea ammonium nitrate, 25 kg N ha−1; UAN_50: liquid urea ammonium nitrate, 50 kg N ha−1. Error bars show standard errors of the mean
By DAE 50, liquid urea ammonium nitrate (50 kg N ha−1) had significantly decreased the AM fungal colonization by 23%, compared with liquid urea ammonium nitrate (25 kg N ha−1) (Fig. 1c). However, neither digestate nor liquid urea ammonium nitrate application affected AM fungal colonization at DAE 75 (Fig. 1c). At DAE 50, hyphal length did not respond to either digestate or liquid urea ammonium nitrate application (Fig. 1d). At DAE 75, hyphal length in the liquid urea ammonium nitrate at 50 kg N ha−1 treatment was significantly decreased by 31% compared with liquid urea ammonium nitrate at 25 kg N ha−1, while there were no significant differences between liquid urea ammonium nitrate at 50 kg N ha−1 and the control treatment (Fig. 1d). Hyphal length following application of digestate at 50 kg N ha−1 was significantly increased (by 29%), whereas digestate amendment rates had no effect on hyphal length (Fig. 1d).
Relative abundance of bacterial phyla
In order to visualize the overall distribution of OTUs at the 97% similarity level, a non-metric multidimensional scaling (NMDS) plot showed that the bacterial rhizosphere community composition was influenced by amendment treatments (Fig. 3). The separation between different amendment treatments indicates that the bacterial rhizosphere communities are dissimilar under these conditions. Subsequent community analysis by PERMANOVA showed that bacterial rhizosphere community composition was influenced mostly by different fertilizer (DAE 25, P = 0.019; DAE 50, P < 0.001; DAE 75: P < 0.001), but not by application rate (Table 3). In addition, the bacterial community composition was influenced by fertilizer (P < 0.001) and harvest time (P < 0.001) (Fig. S4c).
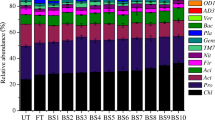
All fertilizer applications strongly affected microbial composition. As shown in Fig. 2, Proteobacteria was the most abundant bacterial phylum across the treatments, accounting for 31, 29, and 29% of all taxa on average at DAE 25, DAE 50, and DAE 75, followed by Firmicutes (18, 18, and 17%), Acidobacteria (14, 15, and 14%), Bacteroidetes (8.9, 7.5, and 7.9%) and Actinobacteria (8.8, 8.8, and 8.3%), but other taxa were present at lower abundance. Application of liquid urea ammonium nitrate decreased the abundance of Acidobacteria and Planctomycetes by 31.2 and 17.8% (DAE 25), 18.0 and 15.2% (DAE 50), and 20.8 and 22.3% (DAE 75), respectively, compared with no fertilizer for the average of the two amendments. Application of liquid urea ammonium nitrate increased the abundance of Firmicutes and Cyanobacteria by 29.0 and 39.1%, respectively, at DAE 75. Digestate significantly increased the relative abundance of Firmicutes and Bacteroidetes by 10.0 and 22.7%, respectively, at DAE 25 for the average of the two rates (Fig. 3). However, digestate amendment did not affect the relative abundance of Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, Chloroflexi Planctomycetes, and Verrucomicrobia at DAE 75 (Fig. 2). At the genus level, the relative abundance of Bacillus belonging to the phylum Firmicutes was the highest, accounting for 8.2, 9.5, and 9.3% of the bacterial composition on average at DAE 25, DAE 50, and DAE 75, followed by Chitinophagaceae (3.7, 4.2, and 4.4%), Kaistobacter (2.8, 2.8, and 2.6%), and Rhodoplanes (1.7, 1.9, and 2.1%), but other taxa were present at lower abundance. The relative abundance of the Bacillus, Rhodococcus, and Streptomyces were greater in liquid urea ammonium nitrate (50 kg N ha−1) compared with those in other treatments at DAE 25 and 50 (Tables S2–S4). In addition, liquid urea ammonium nitrate (50 kg N ha−1) and digestate (50 kg N ha−1) significantly decreased the relative abundance of Rhodoplane, compared with their respective lower rate (Tables S2–S4). In contrast, soil application of liquid urea ammonium nitrate (50 kg N ha−1) and digestate (50 kg N ha−1) resulted in a decrease in the relative abundance of Alicyclobacillus.
Rhizosphere bacterial relative abundance observed at the Phylum level of annual ryegrass assessed at 25, 50, and 75 days after emergence (DAE). The group accounting for ≥ 1% are shown while those < 1% are integrated into ‘other’. See Fig. 1 for treatment descriptions. Error bars are the standard error of the mean
Non-metric multidimensional scaling plot of soil bacterial communities of annual ryegrass assessed at 25 (a), 50 (b), and 75 (c) days after emergence (DAE) using OTU based (97% similarity) Bray-Curtis dissimilarities distance. See Fig. 1 for treatment descriptions
Bacterial OTU richness and diversity
The OTU richness and phylogenetic diversity of rhizosphere soil were influenced by digestate and liquid urea ammonium nitrate (Table 4). Although the rarefaction curves did not plateau, a high proportion of the bacterial population was sampled as inferred by the Good’s coverage estimator score reaching 90 to 96%, 94 to 98%, and 87 to 98% coverage at DAE 25, 50, and 70, respectively. Comparing with lower application rates, bacterial OTU richness significantly increased with increasing amount of digestate addition, while liquid urea ammonium nitrate exhibited the opposite trend at DAE 75. By DAE 50, the digestate (50 kg N ha−1) significantly increased the Shannon index, compared with the lower rate. However, no significant differences among fertilizer treatments were observed for rhizosphere Shannon index at DAE 25 and 75 (Table 4). Furthermore, neither digestate nor liquid urea ammonium nitrate application significantly impacted evenness (Table 4).
Rhizosphere bacterial metabolic profile prediction: PICRUSt
We used the PICRUSt program to predict metagenome functional content based on the Kyoto encyclopedia of genes and genomes (KEGG) classification. The nearest sequenced taxon index (NSTI) was used to quantify the availability of nearby genome representatives for each sample. The accuracy of PICRUSt decreased with increases in NSTI scores. The average NSTI for all samples for the metagenomic predictions was 0.128 ± 0.001 in this study. Our NSTI values are lower than those of previous work (Langille et al. 2013), which revealed higher NSTI values in environmental communities (0.17 ± 0.02). Additionally, there was low variability among all samples (Table S5) and these data were comparable to other soil experiments (Chen et al. 2016).
Putative rhizosphere C cycling genes
Predicted rhizosphere bacterial functional genes responsible for degradation of starch, hemicellulose, cellulose, chitin, and lignin were detected in all rhizosphere soil samples (Fig. 4). Overall, liquid urea ammonium nitrate significantly decreased the predicted abundance of genes coding for beta-galactosidase involved in hemicellulose degradation. At DAE 25, digestate (50 kg N ha−1) significantly decreased the predicted abundance of genes coding for alpha-amylase and glucoamylase involved in starch degradation, xylanase for hemicellulose degradation, and beta-glucosidase for cellulose degradation, compared with the liquid urea ammonium nitrate and control treatments (Fig. 4a). The abundances of predicted genes involved in starch, hemicellulose, cellulose, pectin, and chitin degradation were enhanced with increasing liquid urea ammonium nitrate application at DAE 50 (Fig. 4b). At DAE 75, digestate significantly decreased the predicted abundance of genes coding for alpha-amylase and pullulanase involved in starch degradation, in comparison with the liquid urea ammonium nitrate treatments. However, liquid urea ammonium nitrate significantly decreased the abundance of putative genes associated with arabinofuranosidase and xylanase involved in hemicellulose degradation, endoglucanase, and beta-glucosidase for cellulose degradation and chitinase for chitin degradation. The abundances of putative genes involved in lignin degradation were enhanced by applications of digestate and liquid urea ammonium nitrate fertilization. Most of the detected rhizosphere putative genes were not significantly affected by digestate fertilization (Fig. 4c).
Rhizosphere PICRUSt C degrading gene abundance of annual ryegrass assessed at 25, 50, and 75 days after emergence (DAE). See Fig. 1 for treatment descriptions. Error bars are the standard error of the mean
Putative rhizosphere N cycling genes
Overall, digestate and liquid urea ammonium nitrate addition increased the predicted gene abundance of amoA, amoB, and Hao involved in nitrification, nifH involved in N2 fixation as compared with CK. However, compared with liquid urea ammonium nitrate (50 kg N ha−1), digestate addition (at both rates) significantly decreased amoA, amoB, and Hao abundance (Fig. 5). At DAE 25, the application of liquid urea ammonium nitrate at both rates significantly decreased predicted genes (nosZ and nirS) involved in denitrification. While predicted gene abundance of nosZ involved in denitrification and nirA, narB involved in assimilatory N reduction significantly decreased with increasing digestate addition rate (Fig. 5a). The liquid urea ammonium nitrate treatments enhanced the predicted gene abundances of nrfA and nrfH for dissimilatory N reduction, and ureC involved in urea hydrolysis at DAE 50 and 75, and reduced narB for assimilatory N reduction at DAE 50, nirS for denitrification at DAE 75. The application of digestate did not significantly alter most predicted genes involved in N cycling at DAE 75 (Fig. 5c).
Rhizosphere PICRUSt N cycling gene abundance of annual ryegrass assessed at 25, 50, and 75 days after emergence (DAE). See Fig. 1 for treatment descriptions. Error bars are the standard error of the mean
The diversity of predicted N cycling genes within the rhizosphere increased with both digestate and liquid urea ammonium nitrate fertilization treatments at DAE 75 (Fig. S3c). The digestate treatments decreased the diversity of C cycling genes (Fig. S3d, e and f). To visulaize these effects, a NMDS plot was used to display overall distribution of predicted N reactions in association with different fertilizer and addition rates. Community analysis by PERMANOVA showed that community composition was influenced mostly by fertilizer (P < 0.001), but also by application rate(s) at DAE 25 and 50 (both P < 0.01) with distinct clustering (Fig. S2; Table S1). Communities assessed by C cycling processes showed that the fertilizer had a significant influence, but fertilizer rate had less influence (Fig. S1; Table S1). Furthermore, community analysis by PERMANOVA showed that N and C putative functional genes were significantly influenced by fertilizer (P < 0.001), harvest time (P < 0.001), and their interaction (P < 0.001) (Fig. S4a and b).
Structural equation modeling identifies key linkages among plant, soil, and microbial variables
The integrated responses of the overall soil-microbe system were investigated using structural equation modeling (SEM), which can reveal relationships of soil, microbial, and plants following digestate and liquid urea ammonium nitrate application and rates. The final models of digestate and liquid urea ammonium nitrate demonstrated a good fit to the data (χ2 = 4.08, P = 0.665 and χ2 = 7.85, P = 0.449, respectively). The models were significant at the 0.001 level with R2 = 0.19–0.97. Overall, increasing the digestate rate significantly increased C cycling gene diversity and significantly decreased N cycling gene diversity and bacterial community composition due to increased NO3−-N. Digestate addition directly affected the pH initially and then indirectly affected C cycling gene community composition by decreasing the soil pH. In addition, the AM fungal colonization was significantly correlated with the community composition of bacterial and ryegrass biomass (Fig. 6a). Furthermore, the ryegrass biomass was significantly affected by digestate addition, bacterial community composition, and C cycling gene diversity. Although bacterial diversity did not have significant direct effects on ryegrass biomass, it could indirectly affect biomass by affecting the bacterial community composition. Moreover, the content of NO3−-N, exchangeable NH4+-N in soil, and soil pH directly affected the ryegrass biomass (Fig. 6a). In general, the liquid urea ammonium nitrate addition rate significantly affected bacterial N and C cycling gene diversity and community composition with increased soil NO3−-N, exchangeable NH4+-N, and decreased soil pH. The colonization of roots by AM fungi was significantly correlated with the composition of the bacterial communities and the C and N cycling genes (Fig. 6b). Biomass was significantly directly affected by liquid urea ammonium nitrate addition, AM fungal colonization, NO3−-N, exchangeable NH4+-N, and pH (Fig. 6b).
The effects of soil properties, AMF and the bacterial diversity, richness and community composition on the annual ryegrass biomass estimated using structural equation modeling under digestate (a) and UAN (b) application. Blue lines indicate positive effects, while red lines indicate negative effects. The width of arrows indicates the strength of significant standardized path coefficients (P < 0.05). Solid and dashed lines indicate positive and negative pathways, respectively. AMF: arbuscular mycorrhizal fungi; UAN: liquid urea ammonium nitrate
Discussion
Effects on physicochemical and biological soil properties
Digestate from anaerobic digestion of food wastes has potential use as an organic amendment due to its high content of organic matter and significant amount of exchangeable NH4+ (Buhlmann et al. 2019). The changes in inorganic–N (NO3−-N and exchangeable NH4+-N) in the soil after the application of digestate and liquid urea ammonium nitrate suggest rapid nitrification of the exchangeable NH4+-N added in the digestate and in the liquid urea ammonium nitrate applied to the soil. Digestate and liquid urea ammonium nitrate contain a high proportion of exchangeable NH4+-N, which can be nitrified quickly in soil, and a relatively low quantity of organic forms (Alburquerque et al. 2012b). The initial high NO3−-N concentration in the soil decreased in the successive sampling period, so there was no accumulation of NO3−-N in the soil at the end of the experiment. Nitrate can be taken up directly by plants and incorporated into tissues, but it also has a high potential for entering groundwater through leaching or entering the atmosphere through denitrification (Garcia-Sánchez et al. 2015; Di and Cameron 2016). In addition, a previous study has shown that both exchangeable NH4+ and NO3− can be taken up by AM fungi which can transport N to their host plants (Govindarajulu et al. 2005). AM fungal hyphae are at least three orders of magnitude thinner than roots and can extend more than 10 cm beyond the root surface (Cavagnaro et al. 2005), allowing them to take up nutrients quickly and extensively. AM fungal hyphae may also be able to access N in the less mobile exchangeable NH4+ form, acquiring this form of N before conversion to NO3− (Hodge and Storer 2015). In this experiment, digestate application increased AM fungal hyphal length and reduced the NO3−-N content when compared with liquid urea ammonium nitrate.
Variations in either soil pH or electrical conductivity might affect nutrient availability and uptake, as well as the biomass, activity, and composition of the soil microbial community (Lauber et al. 2009). Indeed, soil pH decreased with the increase in amendment rate throughout the experimental period. Soil organic matter has been recognized to be crucial for improving soil quality and regulating many soil functions (García-Sánchez et al. 2015). Here, we report that the addition of digestate to soil provided easily available organic matter, mostly degradable in the short term, which did not contribute to the increase in the soil total C content (García-Sánchez et al. 2015). Conversely, the content of dissolved organic C in soil treated with digestate increased significantly with harvest time. These findings suggest that an enhancement in dissolved organic C could originate from the release of organic substances during the decomposition of the organic matter within the digestate. This is consistent with a previous study of García-Sánchez et al. (2015) which showed that the application of digestate led to an increase in dissolved organic C. This was probably because digestate application elevated available organic matter which was easily degraded and transformed by soil microbial activity.
Effects of digestate and liquid urea ammonium nitrate on plant growth, AM fungal colonization, and hyphal density
Plant growth showed significant improvements with application of both fertilizer types and rates in this soil which was N deficient for growth of ryegrass (see Methods). Walsh et al. (2012b) also reported that grasses to which digestate was applied had similar or better yields than those receiving inorganic N fertilizers, but this effect would depend on the original soil N. Digestate application, relative to inorganic fertilizer, may also increase soil organic matter and hence improve nutrient retention and soil quality (Alburquerque et al. 2012a).
Our observed reduction of AM fungal colonization with liquid urea ammonium nitrate addition supports previous studies where N-induced declines in AM fungal colonization (e.g. Gryndler et al. 2006; Jiang et al. 2018). This indicates that N enrichment of systems that are not P-limited reduces plant C allocation to mycorrhizal fungi (Johnson 2010). However, our amendment treatments did not reduce the abundance of AM fungal extraradical hyphae in the soil. The previous study showed that N addition reduced the arbuscular and vesicular colonization, but did not reduce the abundance of AM fungal extraradical hyphae (Jiang et al. 2018). Furthermore, our finding of increased development of AM fungal hyphae in soil following digestate application is in agreement with an earlier pot study (Gryndler et al. 2006). Such increased development of AM fungi in soil amended with organic matter could be related to a general increase in soil biological activity, where AM fungi may benefit from the release of other nutrients and growth-stimulating substances (Gryndler et al. 2006). However, in our case, the hyphal density of AM fungi was significantly decreased with the increase in liquid urea ammonium nitrate application rate. Similarly, Ngwene et al. (2013) reported that AM fungal hyphae density decreased with the supply of exchangeable NH4+-N. Therefore, AM fungi could obtain less C allocation from the host and subsequently show lower hyphal density (Zheng et al. 2014).
Effect of digestate and liquid urea ammonium nitrate on rhizosphere bacteria and predicted functional gene
Changes in soil bacterial community composition associated with changes in quantity and quality of soil organic matter are likely to influence soil C storage and N cycling processes (Cusack et al. 2011; Zhang et al. 2019). Previous studies have observed shifts in soil bacterial composition following N (Zeng et al. 2016) or digestate application (Gielnik et al. 2019) to soil. Changes in community composition appeared quickly, being detected by DAE 25. However, rhizosphere microbiome changes were affected by the type of fertilizer (liquid urea ammonium nitrate or digestate) regardless of application rate. There was lower bacterial community richness and diversity in the rhizosphere following the addition of liquid urea ammonium nitrate, which is consistent with previous demonstration of a decline in microbial diversity following N enrichment (Zeng et al. 2016). However, in our study, digestate application did not significantly decrease soil bacterial diversity and richness.
Digestate application increased the input of organic C and N in soil which have been shown to be involved in enhancement of diversity and richness of bacteria (García-Sánchez et al. 2015). Soil pH and exchangeable NH4+-N availability are critical in determining bacterial community composition and diversity (Zeng et al. 2016). Lower soil pH can result in changes in nutrient availability (Stark et al. 2012), which may indirectly affect soil microbial community composition. Using a structural equational model (SEM), we found that the correlation of fertilization indirectly affected rhizosphere bacterial diversity and community by increasing soil NO3−-N. However, other factors may also contribute to soil microbial community composition changes in response to nutrient enrichment. Qin et al. (2016) found that AM fungal hyphae altered bacterial community composition. Our model showed that digestate and liquid urea ammonium nitrate addition significantly changed AM fungal colonization and hyphal growth which in return significantly affected bacterial composition.
DNA sequencing of the rhizosphere bacterial community indicated consistent general phylum-level responses associated with digestate and liquid urea ammonium nitrate addition in the soil. Acidobacteria and Planctomycetes generally decreased in abundance, while Cyanobacteria, Firmicutes, and Actinobacteria increased in abundance following liquid urea ammonium nitrate addition. Shifts in bacterial composition following N manipulation were previously explained by the copiotrophic hypothesis, in which copiotrophic groups (e.g., Firmicutes and Actinobacteria) that have fast growth rates are more likely to increase in nutrient-rich conditions, while oligotrophic groups (e.g., Acidobacteria and Planctomycetes) that have slower growth rate would likely decline (Fierer et al. 2007; Zeng et al. 2016). This shift is consistent with the microbial N mining hypothesis, which suggests that soil microbes reduce decomposition of recalcitrant C in response to lowered N requirement and lead to a shift towards labile C decomposition under N enrichment condition (Craine et al. 2007). In addition, digestate addition also significantly increased the relative abundance of Firmicutes. However, some oligotrophic organisms such as the Cyanobacteria did become more dominant following liquid urea ammonium nitrate addition. This indicated that phylum or class-level responses were mainly determined by changes at a lower taxonomic level, as not all bacterial taxa belonging to the same group shifted in a similar manner (Zeng et al. 2016). Also, bacterial rhizosphere responses were frequently inconsistent, and the response was affected by both the amount of liquid urea ammonium nitrate and digestate added and the duration of the treatment (Janssens et al. 2010). For example, Bacteroidetes was significantly higher in relative abundance with digestate than with liquid urea ammonium nitrate (50 kg N ha−1). Digestate amendment increased the relative abundance of Bacteroidetes at DAE 25, while there was no significant difference at DAE 75. The greater dominance of Bacteroidetes in the digestate treatments may, in part, reflect increased organic C availability (Eilers et al. 2012). Furthermore, the relative abundance of the dominant bacterial phyla was altered shortly after addition of digestate and liquid urea ammonium nitrate treatments, but these changes disappeared with time in the digestate treatment. This is consistent with a previous report that soil amendment with digestate did not have a major impact on soil microbial properties (Podmirseg et al. 2019). Thus, the autochthonous microbiota that prevails in the soil could be outcompeting and partially inhibiting the proliferation of allochthonous microorganisms. However, the long-term effect of digestate management to agricultural soil needs to be further studied.
Three genera more responsive to liquid urea ammonium nitrate addition were Bacillus, Rhodococcus, and Streptomyces. Bacillus and Rhodococcus have been shown to improve nutrient uptake, thus enhancing plant growth (Babalola 2010; Backer et al. 2018), which may have contributed to the higher biomass in the liquid urea ammonium nitrate (50 kg N ha−1) treatment. The genus Streptomyces includes nitrogenase which can play a role in nitrogen fixation (Dahal et al. 2017). In addition, the genus Rhodoplanes may represent important group of free-living nitrogen-fixing bacteria (Buckley et al. 2007). The relative abundance of Rhodoplanes significantly decreased with liquid urea ammonium nitrate input when compared with digestate application. Thus, the genus Rhodoplanes may be suppressed by liquid urea ammonium nitrate addition. However, further studies are needed to demonstrate its potential function for plant growth promotion, which may be useful in agriculture.
The 16S rRNA gene profiling information from PICRUSt (Langille et al. 2013) was used to predict the abundance of C and N functional genes under different fertilizer applications. Fertilization (digestate, liquid urea ammonium nitrate) treatments influenced most predicted rhizosphere genes involved in C degradation and N cycling. Previous studies reported that the function of microbial communities was affected by different fertilization practices (He et al. 2007), and this may accelerate soil C and N turnover. In these studies, the nifH gene plays a key role in the biological conversion of atmospheric N to exchangeable NH4+ or fixed N2 (Fani et al. 2000). Consistent with the enhanced abundance of nifH following N fertilization observed by Zhang et al. (2019), we found an increase in nifH with digestate and liquid urea ammonium nitrate addition at DAE 50 and 75. N-fixing bacilli are often isolated from rhizosphere soil (Achouak et al. 1999) and Bacillus was a most abundant genus whenever digestate and liquid urea ammonium nitrate were applied to soil. In addition, there was a decrease in nifH with increasing levels of liquid urea ammonium nitrate addition. However, in our study, stability in the predicted rhizosphere’s nifH gene abundance was observed after addition of digestate fertilizer. Fertilizer affects the N2 fixing bacteria present in plant rhizospheres in different ways, depending not only on the different fertilizer, but also possibly on the soil type (Li et al. 2014b), nutrient availability (Waldrop and Zak 2006), and soil acidification (Ning et al. 2015).
Nitrification is an important process in N metabolism (Su et al. 2015). The main genes involved include hao, amoA, and amoB. The abundances of amo and Hao increased with digestate and liquid urea ammonium nitrate addition and this was consistent with a previous study (Zhang et al. 2019) where amo- responded positively to exchangeable NH4+-N and NO3−-N enhancement. Although digestate application increased the abundance of amoAB and Hao, their abundance in the rhizosphere was significantly decreased when compared with liquid urea ammonium nitrate addition. Thus, addition of digestate may reduce soil acidification. However, appropriate digestate management and processing practices are needed to avoid potential acidification and eutrophication impacts due to increased nutrient leaching (Alburquerque et al. 2012b) although this is dependent on the local soil quality and meteorological conditions as well as digestate characteristics (Evangelisti et al. 2014).
Liquid urea ammonium nitrate application increased the abundance most of the genes involved in C degradation, suggesting that fertilization can accelerate soil C turnover in this sandy soil. Addition of liquid urea ammonium nitrate and digestate both enhanced the abundance of genes related to soil oxidoreductase (catalase). This may be the consequence of stimulation of both microbial growth and activity via improved nutrient availability as well as changes in microbial community composition induced by fertilizer addition (Ge et al. 2009; Ai et al. 2012). However, the predicted gene abundance in the rhizosphere responsible for chitin and more labile C (arabinofuranosidase, xylanase, beta-galactosidase) degradation was decreased by liquid urea ammonium nitrate application when compared with digestate. This is likely to reduce the content of labile degradable C input in soil (Yang et al. 2020). The digestate had a lesser effect on C degradation genes which is consistent with a previous study (Möller 2015) and may be important in maintaining C stability in soil. However, we only determined the presence of functional genes and not their expression, and we did not quantify enzyme activities. The presence of microbial groups which can carry out a given function in soil cannot be used as proxy of potential microbial functions of soil (Nannipieri et al. 2020). Thus, further research is needed to consider the linkage between microbial functional genes and enzyme activities of soils.
Conclusions
This study demonstrated that digestate added at an equivalent N content to urea (liquid urea ammonium nitrate) was equally effective as a fertilizer for annual ryegrass growth. However, digestate increased the development of AM fungal hyphae density, but the amount of liquid urea ammonium nitrate addition significantly reduced AM fungal hyphae density. The amount of fertilizer application had little influence on the bacterial phylogenetic diversity and composition, and over time, different forms of N fertilizer and application rates can significantly shift rhizosphere bacterial community composition. The abundance of most of the functional genes involved in C and N cycling were significantly stimulated after digestate and liquid urea ammonium nitrate amendment. Although use of digestate had a lesser impact on soil microflora diversity and potential function than the liquid urea ammonium nitrate fertilizer, the community composition and N and C putative functional genes changed with time. Resistance and resilience of the main microbial groups and their activity need to be evaluated. Quantification of the targeted expressed genes would contribute to understanding the function of microbial communities (Nannipieri et al. 2019).
References
Abbott LK, Robson AD (1981) Infectivity and effectiveness of vesicular arbuscular mycorrhizal fungi: effect of inoculum type. Aust J Agric Res 32:631–639
Achouak W, Normand P, Heulin T (1999) Comparative phylogeny of rrs and nifH genes in the Bacillaceae. Inter J Syst Bacteriol 49:961–967
Adhikari BK, Barrington S, Martinez J (2006) Predicted growth of world urban food waste and methane production. Waste Manag Res 24:421–433
Ai C, Liang GQ, Sun JW, Wang XB, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173–174:330–338
Alburquerque JA, de la Fuente C, Bernal MP (2012a) Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric Ecosyst Environ 160:15–22
Alburquerque JA, De la Fuente C, Campoy M, Carrasco L, Nájera I, Baixauli C, Caravaca F, Roldán A, Cegarra J, Bernal MP (2012b) Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur J Agron 43:119–128
Andrews SS, Karlen DL, Cambardella CA (2004) The soil management assessment framework: a quantitative soil quality evaluation method. Soil Sci Soc Am J 68:1945–1962
Andruschkewitsch M, Wachendorf C, Wachendorf M (2013) Effects of digestates from different biogas production systems on above and belowground grass growth and the nitrogen status of the plant-soil-system. Grass Sci 59:183–195
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32:1559–1570
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:1473
Buckley DH, Huangyutitham V, Hsu SF, Nelson TA (2007) Stable isotope probing with 15N2 reveals novel non-cultivated diazotrophs in soil. Appl Environ Microb 73:3196–3204
Buhlmann CH, Mickan BS, Jenkins SN, Tait S, Kahandawala TK, Bahri PA (2019) Ammonia stress on a resilient mesophilic anaerobic inoculum: methane production, microbial community, and putative metabolic pathways. Bioresour Technol 275:70–77
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaug PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 68:1621–1624
Caracciolo AB, Bustamante MA, Nogues I, Di Lenola M, Luprano ML, Grenni P (2015) Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 245:89–97
Cavagnaro TR, Smith FA, Smith SE, Jakobsen I (2005) Functional diversity in arbuscular mycorrhizas: exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ 285:642–650
Chen L, Luo Y, Xu JM, Yu ZY, Zhang KL, Brookes PC (2016) Assessment of bacterial communities and predictive functional profiling in soils subjected to short-term fumigation-incubation. Microb Ecol 72:240–251
Coelho JJ, Hennessy A, Casey I, Woodcock T, Kennedy N (2019) Responses of ryegrass, white clover, soil plant primary macronutrients and microbial abundance to application of anaerobic digestates, cattle slurry and inorganic N-fertiliser. Appl Soil Ecol 144:112–122
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Cusack D, Silver W, Torn M, Burton S, Firestone M (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92:621–632
Dahal B, Nandakafle G, Perkins L, Brözel VS (2017) Diversity of free-living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol Res 195:31–39
Dai XH, Hu CL, Zhang D, Dai L, Duan NN (2017) Impact of a high ammonia-ammonium pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion. Bioresour Technol 245:598–605
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS microbial Eco 72:313–327
DeVries FT, Shade A (2013) Controls on soil microbial community stability under climate change. Front Microbiol 4:265
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sediments 16:1401–1420
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65
Evangelisti S, Lettieri P, Borello D, Clift R (2014) Life cycle assessment of energy from waste via anaerobic digestion: a UK case study. Waste Manag 34:226–237
Fani R, Gallo R, Liò P (2000) Molecular evolution of nitrogen fixation: the evolutionary history of the nifD, nifK, nifE, and nifN genes. J Mol Evol 51:1–11
FAO (2011) Global food losses and food waste-extent, causes and prevention, 2011. Global Food Losses and Food Waste e Extent, Causes and Prevention, SAVE FOOD: Global Initiative on Food Loss and Waste Reduction, Rome
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
García-Sánchez M, Siles JA, Cajthaml T, García-Romera I, Tlustoš P, Száková J (2015) Effect of digestate and fly ash applications on soil functional properties and microbial communities. Eur J Soil Biol 71:1–12
Ge GF, Li ZJ, Zhang J, Wang LG, Xu MG, Zhang JB, Wang JK, Xie XL, Liang YC (2009) Geographical and climatic differences in long-term effect of organic and inorganic amendments on soil enzymatic activities and respiration in field experimental stations of China. Eco Complex 6:421–431
Giacometti C, Demyan MS, Cavani L, Marzadori C, Ciavatta C, Kandeler E (2013) Chemical and microbiological soil quality indicators and their potential to differentiate fertilization regimes in temperate agroecosystems. Appl Soil Ecol 64:32–48
Gianfreda L, Ruggiero P (2006) Enzyme activities in soil. In: Nannipieri P, Smalla K (eds) Nucleic acids and proteins in soil. Soil Biology. Springer, Berlin, pp 257–311
Gielnik A, Pechaud Y, Huguenot D, Cébron A, Riom JM, Guibaud G, Esposito G, van Hullebusch ED (2019) Effect of digestate application on microbial respiration and bacterial communities' diversity during bioremediation of weathered petroleum hydrocarbons contaminated soils. Sci Total Environ 670:271–281
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gryndler M, Larsen J, Hršelová H, Řezáčová V, Gryndlerová H, Kubát J (2006) Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 16:159–166
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesiculararbuscular mycorrhizal fungi associated with Trifolium subterraneum. New Phytol 120:371–380
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jiang SJ, Liu YJ, Luo JJ, Qin MS, Johnson NC, Öpik M, Vasar M, Chai YX, Zhou XL, Mao L, Du GZ, An LZ, Feng HY (2018) Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem. New Phytol 220:1222–1235
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Kempers AJ, Luft AG (1988) Re-examination of the determination of environmental nitrate as nitrite by reduction with hydrazine. Analyst 113:1117–1120
Kibblewhite MG, Ritz K, Swift MJ (2008) Soil health in agricultural systems. Phil. Trans R Soc B 363:685–701
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R, Beiko RG (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-basedassessment of soil pH as a predictor of soil bacterial community structure atthe continental scale. Appl Environ Microbiol 75:5111–5120
Li SS, Du YH, Guo P, Guo LD, Qu KY, He JP (2014a) Effects of different types of N deposition on the fungal decomposition activities of temperate forest soils. Sci Total Environ 497:91–96
Li XZ, Rui JP, Xiong JB, Li JB, He ZL, Zhou JZ, Yannarell AC, Machie RI (2014b) Functional potential of soil microbial communities in the maize rhizosphere. PLoS One 9:e112609
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828
Manoharan L, Kushwaha SK, Ahrén D, Hedlund K (2017) Agricultural land use determines functional genetic diversity of soil microbial communities. Soil Biol Biochem 115:423–432
Melikoglu M, Lin C, Webb C (2013) Analysing global food waste problem: pinpointing the facts and estimating the energy content. Open Eng 3:157
Mickan BS, Abbott LK, Fan JW, Hart MM, Siddique KH, Solaiman ZM, Jenkins SN (2018) Application of compost and clay under water-stressed conditions influences functional diversity of rhizosphere bacteria. Biol Fert soils 54:55–70
Mickan BS, Abbott LK, Solaiman ZM, Mathes F, Siddique KH, Jenkins SN (2019) Soil disturbance and water stress interact to influence arbuscular mycorrhizal fungi, rhizosphere bacteria and potential for N and C cycling in an agricultural soil. Biol Fert soils 55:53–66
Möller K (2015) Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron Sustain Dev 35:1021–1041
Möller K, Stinner W, Deuker A, Leithold G (2008) Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr Cycl Agroecosyst 82:209–232
Nannipieri P, Penton CR, Purahong W, Schloter M, van Elsas JD (2019) Recommendations for soil microbiome analyses. Biol Fertil Soils 55:765–766
Nannipieri P, Ascher-Jenull J, Ceccherini MT, Pietramellara G, Renella G, Schloter M (2020) Beyond microbial diversity for predicting soil functions: a mini review. Pedosphere 30:5–17
Ngwene B, Gabriel E, George E (2013) Influence of different mineral nitrogen sources (NO3−-N vs. NH4+-N) on arbuscular mycorrhiza development and N transfer in a Glomusintraradices–cowpea symbiosis. Mycorrhiza 23:107–117
Nielsen UN, Ayres E, Wall DH, Bardgett RD (2011) Soil biodiversity and carbon cycling: a review and synthesis of studies examining diversity–function relationships. Eur J Soil Sci 62:105–116
Ning QS, Gu Q, Shen JP, Lv XT, Yang JJ, Zhang XM, He JZ, Huang JH, Wang H, Xu ZH, Han XG (2015) Effects of nitrogen deposition rates and frequencies on the abundance of soil nitrogen-related functional genes in temperate grassland of northern China. J Soils Sediments 15:694–704
Nõlvak H, Truu M, Kanger K, Tampere M, Espenberg M, Loit E, Raave H, Truu J (2016) Inorganic and organic fertilizers impact the abundance and proportion of antibiotic resistance and integron-integrase genes in agricultural grassland soil. Sci Total Environ 562:678–689
Phillips ML, Weber SE, Andrews LV, Aronson EL, Allen MF, Allen EB (2019) Fungal community assembly in soils and roots under plant invasion and nitrogen deposition. Fungal Ecol 40:107–117
Podmirseg SM, Waldhuber S, Knapp BA, Insam H, Goberna M (2019) Robustness of the autochthonous microbial soil community after amendment of cattle manure or its digestate. Biol Fert soils 55:565–576
Qin H, Brookes PC, Xu J (2016) Arbuscular mycorrhizal fungal hyphae alter soil bacterial community and enhance polychlorinated biphenyls dissipation. Front Microbial 7:939
Ramirez KS, Craine JM, Noah F (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18:1918–1927
Sapp M, Harrison M, Hany U, Charlton A, Thwaites R (2015) Comparing the effect of digestate and chemical fertiliser on soil bacteria. Appl Soil Ecol 86:1–9
Schloter M, Nannipieri P, Sørensen SJ, van Elsas JD (2018) Microbial indicators for soil quality. Biol Fertil Soils 54:1–10
Scholer A, Jacquiod S, Vestergaard G, Schulz S, Schloter M (2017) Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol Fertil Soils 53:485–489
Searle PL (1984) The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. Rev Anal 109:549–568
Sigurnjak I, Vaneeckhaute C, Michels E, Ryckaert B, Ghekiere G, Tack FMG, Meers E (2017) Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci Total Environ 599:1885–1894
Stark S, Eskelinen A, Männistö MK (2012) Regulation of microbial community composition and activity by soil nutrient availability, soil pH, and herbivory in the Tundra. Ecosystems 15:18–33
Stott DE, Andrews SS, Liebig MA, Wienhold BJ, Karlen DL (2010) Evaluation of β-glucosidase activity as a soil quality indicator for the soil management assessment framework. Soil Sci Soc Am J 74:107–119
Su JQ, Ding LJ, Xue K, Yao HY, Quensen J, Bai SJ, Wei WX, Wu JS, Zhou J, Tiedje JM, Zhu YG (2015) Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol Ecol 24:136–150
Tampio E, Salo T, Rintala J (2016) Agronomic characteristics of five different urban waste digestates. J Environ Manag 169:293–302
Vestergaard G, Schulz S, Schöler A, Schloter M (2017) Making big data smart—how to use metagenomics to understand soil quality. Biol Fertil Soils 53:479–484
Waldrop MP, Zak DR (2006) Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9:921–933
Walsh JJ, Rousk J, Edwards-Jones G, Jones DL, Williams AP (2012a) Fungal and bacterial growth following the application of slurry and anaerobic digestate of livestock manure to temperate pasture soils. Biol Fert Soils 48:889–897
Walsh JJ, Jones DL, Edwards-jones G, Williams AP (2012b) Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J Plant Nutr Soil Sci 175:840–845
Yang SH, Zheng QS, Yang YF, Yuan MT, Ma XY, Chiariello NR, Docherty KM, Field CB, Gutknecht JLM, Hungate BA, Niboyet A, Le Roux X, Zhou JZ (2020) Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning. Glob Change Biol 26:431–442
Zarezadeh S, Moheimani N, Jenkins S, Hülsen T, Riahi H, Mickan BS (2019) Microalgae and phototrophic purple bacteria for nutrient recovery from agri-industrial effluents: influences on plant growth, rhizosphere bacteria and putative carbon and nitrogen cycling genes. Front Plant Sci 10:1193
Zeng J, Liu XJ, Song L, Lin XG, Zhang HY, Shen CC, Chu HY (2016) Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol Biochem 92:41–49
Zhang J, Kobert K, Flouri T, Stamatakis A (2014) PEAR: a fast and accurate illumina paired-end reAd mergeR. Binformatics 30:614–620
Zhang C, Song ZL, Zhuang DC, Wang J, Xie SS, Liu GB (2019) Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland. Biol Fert Soils 55:229–242
Zheng Y, Kim YC, Tian XF, Chen L, Yang W, Gao C, Song MH, Xu XL, Guo LD (2014) Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS microbial Ecol 89:594–605
Zhu B, Gutknecht JL, Herman DJ, Keck DC, Firestone MK, Cheng W (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192
Acknowledgments
We thank Mr. Geoff Richards for overarching support, laboratory manager Maria Sevo, and technical assistant Natasha Bielawski from Richgro for soil and plant analysis. The visit to UWA by A-T R was funded by the China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1259 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ren, AT., Abbott, L.K., Chen, Y. et al. Nutrient recovery from anaerobic digestion of food waste: impacts of digestate on plant growth and rhizosphere bacterial community composition and potential function in ryegrass. Biol Fertil Soils 56, 973–989 (2020). https://doi.org/10.1007/s00374-020-01477-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-020-01477-6