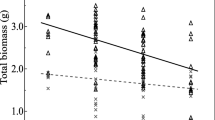
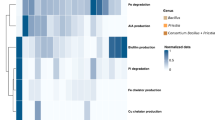
Abstract
Biotic interactions affect the impact of potential plant growth promoting microorganisms in the rhizosphere, but their magnitude and fundamentals are often hardly known. In a pot experiment, two physiologically different strains of the ectomycorrhizal fungus Paxillus involutus (GUL and FRA) were tested separately and in combination with associated bacteria (Sphingomonas paucimobilis 1 L, Ralstonia pickettii 16 B, Sphingomonas sp. 23 L) on their effects on the growth of willows (Salix viminalis). Both P. involutus strains significantly increased the growth of the willows compared with non-inoculated plants, but the magnitude of this effect was significantly affected by the fungal strain. The P. involutus strain GUL with higher synthesis of auxin-like substances, acid phosphatases, siderophores and faster utilisation of yeast extract in vitro increased the willow growth in situ more effectively than strain FRA. Additionally, dual inoculation with the P. involutus strains GUL and FRA in combination with the associated bacteria promoted the willow growth, especially the combination with S. paucimobilis 1 L. This bacterial strain used effectively C sources which are common components of plant root exudates, e.g. glucose, sucrose, maltose and mannose as well as compounds synthesized by fungi, e.g. trehalose in vitro. We conclude that the analyses of fungal metabolites and of C source use of associated bacteria can successfully contribute to accelerate the selection of capable plant growth promoting combinations.





Similar content being viewed by others
References
Abuzindah RA, Read DJ (1986) The role of proteins in the nitrogen nutrition of ectomycorrhizal fungi. New Phytol 103:481–493
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evalutate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Baldarian P (2009) Ectomycorrhizal fungi and their enzymes in soils: is there enough evidence for their role as facultative soil saprotrophs? Oecologia 161:657–660
Barea JM, Gryndler M, Lemanceau P, Schüepp H, Azcón R (2002) The rhizosphere of mycorrhizal plants. In: Gianinazzi S, Schüepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. Birkäuser Verlag, Basel, pp 1–18
Baum C, Hrynkiewicz K, Leinweber P, Meissner R (2006) Heavy metal mobilization and uptake by mycorrhizal and non-mycorrhizal willows (Salix × dasyclados). J Plant Nutr Soil Sci 169:516–522
Baum C, Toljander YK, Eckhardt K-U, Weih M (2009) The significance of host-fungus combinations in ectomycorrhizal symbioses in the chemical quality of willow foliage. Plant Soil. doi:10.1007/s11104-009-9928-x
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98:971–981
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154(2):275–304
Buyer JS, Leong J (1986) Iron transport mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J Biol Chem 261:791–794
Cai YJ, Buswell JA, Chang ST (1994) Production of cellulases and hemicellulases by the straw mushroom, Volvarriella volvacea. Mycol Res 98(9):1019–1024
Cairney JWG (1999) Intraspecific physiological variations for understanding functional diversity in ectomycorrhizal fungi. Mycorrhiza 9:125–135
Cairney JWG, Burke RM (1996) Physiological heterogeneity within fungal mycelia: an important concept for a functional understanding of the ectomycorrhizal symbiosis. New Phytol 134:685–695
Cline GR, Reid CPP, Powell P, Szaniszlo PJ (1984) Effects of a hydroxamate siderophore on iron absorption by Sunflower and Sorghum. Plant Physiol 76:36–39
Colpaert JV, Van Laere A (1996) A comparison of the extracellular enzyme activities of two ectomycorrhizal and a leaf-saprotrophic basidimycete colonizing beech leaf litter. New Phytol 133:133–141
Courty PE, Pouysègur R, Buèe M, Garbaye J (2006) Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol Biochem 38:1219–1222
Courty PE, Bréda N, Garbaye J (2007) Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol Biochem 39:1655–1663
Cullings K, Ishkhanova G, Henson J (2008) Defoliation effects on enzyme activities of the ectomycorrhizal fungus Suillus granulatus in a Pinus contorta (lodgepole pine) stand in Yellowstone National Park. Oecologia 158:77–83
Dahm H, Hrynkiewicz K, Strzelczyk E, Wrótniak W (2003) Studies on the production of siderophores by forest trees endophytic bacteria and fungi. Folia Forest Polon, Ser A-Forestry 45:6–13
de Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811
Dighton J (1991) Acquisition of nutrients from organic resources by mycorrhizal autotrophic plants. Experientia 47:362–369
Dorsch M, Stackebrandt E (1992) Some modifications in the procedure of direct sequencing of PCR-amplified 16S-rDNA. J Microbiol Methods 16:271–279
Dunstan WA, Malajczuk N, Dell B (1998) Effects of bacteria on mycorrhizal development and growth of container grown Eucalyptus diversicolor F. Muell Seedlings Plant Soil 201:241–249
Duponnois R, Plenchette C (2003) A mycorrhiza helper bacterium enhances ectomycorrhizal and endomycorrhial symbiosis of Australian Acacia species. Mycorrhiza 13:85–91
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:83–791
Frey P, Frey-Klett P, Garbaye J, Berge O, Heulin T (1997) Metabolic and gentypic fingerprinting of fluorescent pseudomonads associated with the Douglas Fir—Laccaria bicolor mycorrhzosphere. Appl Environ Microbiol 63:1852–1860
Frey-Klett P, Chavatte M, Clausse M-L, Courrier S, Le Roux C, Raaijmakers MMG, Pieerat J-C, Garbaye J (2005) Ectomycorrhizal symbiosis affects the functional diversity of rhizosphere fluorescent pseudomonads. New Phytol 165:317–328
Furukawa T, Koga J, Adachi T, Kishi K, Syono K (1996) Efficient conversion of l-tryptophan to indole-3-acetic acid and/or tryptophanol by some species of Rhizoctonia. Plant Cell Physiol 37(7):899–905
Gafur A, Schützendübel A, Langenfeld-Heyser R, Fritz E, Poole A (2004) Compatible and incompetent Paxillus involutus isolates for ectomycorrhiza formation in vitro with poplar (Populus x canescens) differ in H2O2 production. Plant Biol 6:91–99
Garau G, Castaldi P, Santona L, Deiana P, Melis P (2007) Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 142:47–57
Garbaye J (1994) Helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol 128:197–210
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity of basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gascuel O (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14:685–695
Gryndler M, Gryndler M, Hrselová H, Striteskà D (2000) Effect of soil bacteria on growth of hyphae of the arbuscular mycorrhizal (AM) fungus Glomus claroideum. Folia Microbiol 45:545–551
Hahn C, Agerer R (1999) Studium zum Paxillus involutus Formenkreis. Nova Hedwigia 69:241–310
Haselwandter K, Winkelmann G (2002) Ferricrocin—an ectomycorrhizal siderophore of Cenococcum geophilum. Biometals 15:73–77
Hazen GG, Hause JA, Hubicki JA (1965) An automated system for the quantitative determination of proteolytic enzymes using azocasein. Ann N Y Acad Sci 130:761–768
Hedh J, Samson P, Erland S, Tunlid A (2008) Multiple genealogies and species recognition in the ectomycorrhizal fungus Paxillus involutus. Mycol Res 112:965–975
van der Heijden EW, Vries FW, Kuyper TW (1999) Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems. I. Above-ground and below-ground views of ectomycorrhizal fungi in relation to soil chemistry. Can J Bot 77:1821–1832
Hilszczańska D, Ciesielska A, Sierota Z (2008) Enzymatic activity of Thelephora terrestris and Hebeloma crustuliniformae in cultures and mycorrhizal association with Scots pine seedlings. Polish J Environ Stud 17(6):881–886
Hrynkiewicz K, Baum C, Leinweber P (2009a) Mycorrhizal community structure, microbial biomass P and phosphatase activities under Salix polaris as influenced by nutrient availability. Eur J Soil Biol 45:168–175
Hrynkiewicz K, Baum C, Niedojadło J, Dahm H (2009b) Promotion of mycorrhiza formation and growth of willows by the bacterial strain Sphingomonas sp. 23L on fly ash. Biol Fert Soil 45(4):385–394
Hu X, Boyer GL (1996) Siderophore-mediated aluminium uptake by Bacillus megaterium ATCC 19213. Appl Environ Microbiol 62:4044–4048
Jarosch M, Bresinsky A (1999) Speciation and phylogenetic distances within Paxillus s str. (Basidiomycetes, Boletales). Plant Biol 1:701–706
Karabaghli C, Frey-Klett P, Sotta M, Bonnet M, Le Tacon F (1998) In vitro effects of Laccaria bicolor S238N and Pseudomonas fluorescens strain BBc6 on rooting of de-rooted shoot hypocotyls of Norway spruce. Tree Physiol 18:103–111
Kataoka R, Futai K (2009) A new mycorrhizal helper bacterium, Ralstonia species, in the ectomycorrhizal symbiosis between Pinus thunbergii and Suillus granulatus. Biol Fertil Soils 45:315–320
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nuc Acids Res 33:511–518
Kieliszewska-Rokicka B (1992) Acid phosphatase activity in mycorrhizal and non-mycorrhizal Scots pine seedlings in relation to nitrogen and phosphorus nutrition. Acta Soc Bot Pol 61:253–264
Kieliszewska-Rokicka B, Rudawska M, Leski T (1994) The effect of aluminium on the mycelial growth and the acid phosphatase activity at ectomycorrhizal symbiosis of Scots pine (Pinus sylvestris L.). Institute of Dendrology, PAN, Poland, pp 471–479
Kottke I, Guttenberger M, Hampp R, Oberwinkler F (1987) An in vitro method for establishing mycorrhizae on coniferous tree seedlings. Trees 1:191–194
Laiho O (1970) Paxillus involutus as a mycorrhizal symbiont of forest trees. Acta Forest Fenn 106:1–73
Le Quéré A, Schützendübel A, Rajashekar B, Canbäck B, Hedh J, Erland S, Johansson T, Tunlid A (2004) Divergence in gene expression related to variation in host specificity of an ectomycorrhizal fungus. Mol Ecol 13(12):3809–3819
Leake JR, Donnelly DP, Boddy L (2002) Interactions between ecto-mycorrhizal and saprotrophic fungi. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 345–372
Lindahl BD, Finlay RD, Cairney JWG (2005) Enzymatic activities of mycelia in mycorrhizal fungal communities. In: Dighton J, Oudemans J, White J (eds) The fungal community: its organization and role in the ecosystem. Marcel Dekker, New York, pp 331–348
Ma L, Wu X, Zheng L (2009) Relationship between plant hormone level excreted by ectomycorrhizal fungi and growth of poplar NL-895. Front For China 4:236–241
Martin F, Aerts A, Ahren D, Brun A, Danchin EGJ, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V, Salamov A, Shapiro HJ, Wuyts J, Blaudez D, Buee M, Brokstein P, Canback B, Cohen D, Courty PE, Coutinho PM, Delaruelle C, Detter JC, Deveau A, Difazio S, Duplessis S, Fraissinet-Tachet L, Lucic E, Frey-Klett P, Fourrey C, Feussner I, Gay G, Grimwood J, Hoegger PJ, Jain P, Kilaru S, Labbe J, Lin YC, Legue V, Le Tacon F, Marmeisse R, Melayah D, Montanini B, Muratet M, Nehls U, Niculita-Hirzel H, Secq OLMP, Peter M, Quesneville H, Rajashekar B, Reich M, Rouhier N, Schmutz J, Yin T, Chalot M, Henrissat B, Kues U, Lucas S, Van de Peer Y, Podila GK, Polle A, Pukkila PJ, Richardson PM, Rouze P, Sanders IR, Stajich JE, Tunlid A, Tuskan G, Grigoriev IV (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452:88–92
Mueller JG, Devereux R, Santavy DL, Lantz SE, Willis SG, Pritchard PH (1997) Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Van Leeuwenhoek 71:329–343
Niemi K, Scagel CF (2007) Root induction of Pinus sylvestris L. hypocotyls cuttings using specific ectomycorrhizal fungi in vitro. In: Jain SM, Haggman H (eds) Protocols for micropropagation of woody trees and fruits. Springer, Berlin, pp 147–152
Niemi K, Salonen M, Ernsten A, Heinonen-Tanski H, Häggman H (2000) Application of ectomycorrhizal fungi in rooting of Scots pine fascicular shoots. Can J For Res 30:1221–1230
Niemi K, Vuorinen T, Ernstsen A, Häggman H (2002) Ectomycorrhizal fungi and exogenous auxins influence root and mycorrhiza formation of Scots pine hypocotyl cutting in vitro. Tree Physiol 22:1231–1239
Niemi K, Scagel C, Haggman H (2004) Application of ectomycorrhizal fungi in vegetative propagation of conifers. Plant Cell Tiss Organ Cult 78:83–91
Normand L, Bartschi H, Debaud JC, Gay G (1996) Rooting and aclimatization of micropropagated cuttings of Pinus pinaster and Pinus sylvestris are enhanced by the ectomycorrhizal fungus Hebeloma cylindrosporum. Physiol Plant 98:759–766
Poole EJ, Bending GD, Whipps JM, Read DJ (2001) Bacteria associated with Pinus sylvestris–Lactarius rufus ectomycorrhizas and their effects on mycorrhiza formation in vitro. New Phytol 151:741–753
Prabhu V, Biolchini PF, Boyer GL (1996) Detection and identification of ferricrocin produced by ectendomycorrhizal fungi in the genus Wilcoxina. Biometals 9:229–234
Rangel-Castro JI, Levenfors JJ, Danell E (2002) Physiological and genetic characterisation of fluorescent Pseudomonas associated with Cantharellus cibarius. Can J Microbiol 48:739–748
Redlak K, Dahm H, Ciesielska A, Strzelczyk E (2001) Enzymatic activity of ectendomycorrhizal fungi. Biol Fertil Soils 33:83–90
Redlak-Hrynkiewicz K, Dahm H, Ciesielska A (2002) Effect of mycorrhization helper bacteria and root pathogenic fungi on growth of ecto- and ectendomycorrhizal fungi. Phytopathol Pol 23:17–30
Rillig MC (2004) Arbuscular mycorrhizae, glomalin and soil quality. Can J Soil Sci 84:355–363
Rudawska M (1983) The effect of nitrogen and phosphorus on auxin and cytokinin production by mycorrhizal fungi. Arboretum Kórnickie 28:219–236
Rudawska M, Kieliszewska-Rokicka B (1997) Mycorrhizal formation by Paxillus involutus strains in relation to their IAA-synthesizing activity. New Phytol 137:509–517
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Scagel CF, Linderman RG (2000) Changes in root IAA content and growth of bareroot conifers treated with plant growth regulating substances at planting. J Environ Hort 18:99–107
Scagel CF, Linderman RG (2001) Modification of root IAA, plant growth, and survival by application of plant growth regulating substances to container-grown conifers. New Forest 21:159–186
Sherwood RT (1966) Pectin lyase and polygalacturonase production by Rhizoctonia solani and other fungi. Phytopathol 56:279–286
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, London
Strzelczyk E, Pokojska A, Kampert M (1992) The effect of pH on production of plant growth regulators by mycorrhizal fungi. Symbiosis 14:201–215
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4b10. Sinauer Associates, Sunderland
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Tibbett M, Chambers SM, Cairney JWG (1998) Methods for determinating extracellular and surface-bound phosphate activities in ectomycorrhizal fungi. In: Varma A (ed) Mycorrhiza manual. Springer, New York, pp 217–226
Timonen S, Jørgensen KS, Haahtela K, Sen R (1998) Bacterial community structure at defined locations of Pinus sylvestris-Suillus bovinus and -Paxillus involutus mycorrhizopheres in dry pine forest humus and nursery peat. Can J Microbiol 44:499–513
Vonderwell JD, Enebak SA (2000) Differential effects of rhizobacterial strain and dose on the ectomycorrhizal colonization of loblolly Pine seedlings. Forest Sci 46:437–441
Wallander H, Söderström B (1999) Paxillus. In: Cairney JWG, Chambers SM (eds) Ectomycorrhizal fungi key genera in profile. Springer, Berlin, pp 231–252
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. In: Innis MA, Gelfand DH, Sninsky JN, White TJ (eds) PCR Protocols: a guide to methods and applcations. Academic Press, New York, pp 315–322
Winkelmann G (2007) Ecology of siderophores with special reference to the fungi. Biometals 20:379–392
Woodward AW, Bartel B (2005) Auxin: regulation, action and interaction. Ann Bot 95:707–735
Zimmer D, Baum C, Leinweber P, Hrynkiewicz K, Meissner R (2009) Associated bacteria increase the phytoextraction of cadmium and zink from a metal-contaminated soil by mycorrhizal willows. Intern J Phytorem 11:200–213
Acknowledgements
This investigation was supported by a Marie Curie Reintegration Grant financed by the European Commission (MYCOHELPER, MERG-CT-2004-006315). The work of C. Baum was financially supported by a grant from the Federal Ministry of Education and Research (BMBF, Germany), contract 02WT0870. The bacterial strains were kindly provided by Dr. W. Wrótniak (Department of Microbiology, N. Copernicus University of Torun, Poland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrynkiewicz, K., Ciesielska, A., Haug, I. et al. Ectomycorrhiza formation and willow growth promotion as affected by associated bacteria: role of microbial metabolites and use of C sources. Biol Fertil Soils 46, 139–150 (2010). https://doi.org/10.1007/s00374-009-0419-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0419-2