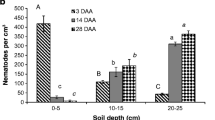
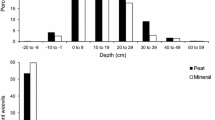
Abstract
The nematophagous fungi Arthrobotrys oligospora and Myzocytiopsis glutinospora increase to large numbers (>103 propagules/g of soil) when moth larvae killed by entomopathogenic nematodes are added to soil microcosms. In spite of these increases, it is unclear how effective these nematophagous fungi are in suppressing nematodes. We measured nematode mortality in microcosms with small numbers of assay nematodes, and we examined assay nematodes recovered at the end of the experiment for signs of fungal parasites. Because the microcosms did not have a moat or other refuge, the assay nematodes remained vulnerable for the 3 days that they were in the soil. Mortality in this experiment was not substantially increased compared to a previous experiment, which measured the mortality of a larger number of assay nematodes in microcosms surrounded by a moat. Mortality, however, increased from 34 to 50% when recovered assay nematodes were examined and when those with conidia of the nematophagous fungus Hirsutella rhossiliensis were considered dead. The zoosporic fungus M. glutinospora was not detected, perhaps because the soil water potential was too low. Contrary to our expectations, there was no evidence of negative feedback on nematodes (i.e., no evidence of density-dependent mortality) because the addition of dead moth larvae greatly increased numbers of resident nematodes and A. oligospora but did not greatly affect the probability of nematode mortality.



Similar content being viewed by others
References
Cooke RC, Godfrey BES (1964) A key to the nematode-destroying fungi. Trans Br Mycol Soc 47:61–74
Farrell FC, Jaffee BA, Strong DR (2006) The nematode-trapping fungus Arthrobotrys oligospora in soil of the Bodega Marine Reserve: distribution and dependence on nematode-parasitized moth larvae. Soil Biol Biochem 38:1422–1429
Jaffee BA (2003a) Negative feedback about density-dependent regulation. Soil Biol Biochem 35:195
Jaffee BA (2003b) Correlations between most probable number and activity of nematode-trapping fungi. Phytopathology 93:1599–1605
Jaffee BA (2004a) Do organic amendments enhance the nematode-trapping fungi Dactylellina haptotyla and Arthrobotrys oligospora? J Nematol 36:267–275
Jaffee BA (2004b) Wood, nematodes, and the nematode-trapping fungus Arthrobotrys oligospora. Soil Biol Biochem 36:1171–1178
Jaffee BA (2006) Interactions between a soil organic amendment, nematodes, and the nematode-trapping fungus Dactylellina candidum. Phytopathology 96:1388–1396
Jaffee BA, Muldoon AE (1995) Numerical responses of the nematophagous fungi Hirsutella rhossiliensis, Monacrosporium cionopagum, and M. ellipsosporum. Mycologia 87:643–650
Jaffee BA, Muldoon AE, Tedford EC (1992a) Trap production by nematophagous fungi growing from parasitized nematodes. Phytopathology 82:615–620
Jaffee B, Phillips R, Muldoon A, Mangel M (1992b) Density-dependent host-pathogen dynamics in soil microcosms. Ecology 73:495–506
Jaffee BA, Strong DR (2005) Strong bottom-up and weak top-down effects in soil: nematode-parasitized insects and nematode-trapping fungi. Soil Biol Biochem 37:1011–1021
Jaffee BA, Strong, DR, Muldoon AE (1996) Nematode-trapping fungi of a natural shrubland: Tests for food chain involvement. Mycologia 88:554–564
Jaffee BA, Tedford EC, Muldoon AE (1993) Tests for density-dependent parasitism of nematodes by trapping and endoparasitic fungi. Biol Control 3:329–336
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey L (ed) Manual of techniques in insect pathology. Academic, San Diego, USA, pp 281–324
Klee AJ (1993) A computer program for the determination of most probable number and its confidence limits. J Microbiol Methodsods 18:91–98
Koppenhöfer AM, Jaffee BA, Muldoon AE, Strong DR (1997) Suppression of an entomopathogenic nematode by the nematode-trapping fungi Geniculifera paucispora and Monacrosporium eudermatum as affected by the fungus Arthrobotrys oligospora. Mycologia 89:557
Koppenhöfer AM, Jaffee BA, Muldoon AE, Strong, DR, Kaya HK (1996) Effect of nematode-trapping fungi on an entomopathogenic nematode originating from the same field site in California. J Invertebr Pathol 68:246–252
Linford MB, Yap F, Oliveira JM (1938) Reduction of soil populations of the root-knot nematode during decomposition of organic matter. Soil Sci 45:127–141
Preisser EL (2003) Field evidence for a rapidly cascading underground food web. Ecology 84:869–874
Preisser EL, Dugaw CJ, Dennis B, Strong DR (2005) Long-term survival of the entomopathogenic nematode Heterorhabditis marelatus. Environ Entomol 34:1501–1506
Strong DR (2002) Populations of entomopathogenic nematodes in foodwebs. In: Gaugler R (ed) Entomopathogenic nematology. CAB International, Oxford, UK, pp 225–240
Strong DR, Kaya HK, Whipple AV, Child AL, Kraig S, Bondonno M, Dyer K, Maron JL (1996) Entomopathogenic nematodes: Natural enemies of root-feeding caterpillars on bush lupine. Oecologia 108:167–173
Strong DR, Maron JL, Connors PG, Whipple A, Harrison S, Jefferies RL (1995) High mortality, fluctuation in numbers, and heavy subterranean insect herbivory in bush lupine, Lupinus arboreus. Oecologia 104:85–92
Timper P, Kaya HK (1989) Role of the second-stage cuticle of entomogenous nematodes in preventing infection by nematophagous fungi. J Invertbr Pathol 54:314–321
Timper P, Kaya HK, Jaffee BA (1991) Survival of entomogenous nematodes in soil infested with the nematode-parasitic fungus Hirsutella rhossiliensis (Deuteromycotina:Hyphomycetes). Biol Control 1:42–50
Yeates GW, Bongers T, de Goede RGM, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera-an outline for soil ecologists. J Nematol 25:315–331
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaffee, B.A., Bastow, J.L. & Strong, D.R. Suppression of nematodes in a coastal grassland soil. Biol Fertil Soils 44, 19–26 (2007). https://doi.org/10.1007/s00374-007-0174-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-007-0174-1