Abstract
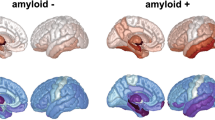
Tau tangles in the brain cortex spread along the brain network in distinct patterns among Alzheimer's patients. We aim to simulate their network-based spreading within the cortex, tailored to each individual along the Alzheimer's continuum, without assuming any assumptions about the network architecture. A group-level intrinsic spreading network was constructed to model the pathways for the proximal and distal spreading of tau tangles by optimizing the biophysical model based on a discovery dataset of longitudinal tau positron emission tomography images for 78 amyloid-positive individuals. Group-level spreading parameters were also obtained and subsequently adjusted to produce individuated tau trajectories. By simulating these individuated tau spreading models for every individual in the discovery dataset, we successfully captured proximal and distal tau spreading, allowing reliable inferences about the underlying mechanism of tau spreading. Simulating the models also allowed highly accurate prediction of future tau topography for both discovery and independent validation datasets.








Similar content being viewed by others
Data availability
The discovery dataset generated and/or analyzed during the current study is not publicly available due to confidentiality agreements but is available from the corresponding author upon reasonable request. The validation dataset is available in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) repository (https://ida.loni.usc.edu/) and also available from the corresponding author upon reasonable request.
Code availability
All original code is available in this paper’s supplementary information.
References
Brunello CA et al (2020) Mechanisms of secretion and spreading of pathological tau protein. Cell Mol Life Sci 77:1721–1744
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12(6):609–622
Bejanin A et al (2017) Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 140(12):3286–3300
De Calignon A et al (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73(4):685–697
Hu W et al (2016) Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimers Dement 12(10):1066–1077
Takeda S et al (2015) Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat Commun 6(1):8490
DeVos SL et al (2018) Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease brain. Front Neurosci 12:267
Zanier ER et al (2018) Induction of a transmissible tau pathology by traumatic brain injury. Brain 141(9):2685–2699
Charil A et al (2019) Tau subtypes of Alzheimer’s disease determined in vivo using flortaucipir PET imaging. J Alzheimers Dis 71(3):1037–1048
Vogel JW et al (2021) Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat Med 27(5):871–881
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Schäfer A, Mormino EC, Kuhl E (2020) Network diffusion modeling explains longitudinal tau pet data. Front Neurosci 14:566876
Yang F et al (2021) Longitudinal predictive modeling of tau progression along the structural connectome. Neuroimage 237:118126
Therriault J et al (2022) Intrinsic connectivity of the human brain provides scaffold for tau aggregation in clinical variants of Alzheimer’s disease. Sci Transl Med 14(659):eabc8693
Cornblath EJ et al (2021) Computational modeling of tau pathology spread reveals patterns of regional vulnerability and the impact of a genetic risk factor. Sci Adv 7(24):eabg6677
Fornari S et al (2019) Prion-like spreading of Alzheimer’s disease within the brain’s connectome. J R Soc Interface 16(159):20190356
Putra P et al (2021) Braiding Braak and Braak: Staging patterns and model selection in network neurodegeneration. Netw Neurosci 5(4):929–956
Raj A, Kuceyeski A, Weiner M (2012) A network diffusion model of disease progression in dementia. Neuron 73(6):1204–1215
Wu JW et al (2013) Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem 288(3):1856–1870
Franzmeier N et al (2020) Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat Commun 11(1):347
Franzmeier N et al (2019) Functional connectivity associated with tau levels in ageing, Alzheimer’s, and small vessel disease. Brain 142(4):1093–1107
Ossenkoppele R et al (2019) Tau covariance patterns in Alzheimer’s disease patients match intrinsic connectivity networks in the healthy brain. NeuroImage Clin 23:101848
Brown JA et al (2019) Patient-tailored, connectivity-based forecasts of spreading brain atrophy. Neuron 104(5):856-868.e5
Franzmeier N et al (2020) Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Sci Adv 6(48):eabd1327
Schäfer A, et al (2022) Correlating tau pathology to brain atrophy using a physics-based Bayesian model. Eng Comput, pp 1–11
Schäfer A et al (2021) Bayesian physics-based modeling of tau propagation in Alzheimer’s disease. Front Physiol 12:702975
Rao AV (2014) Trajectory optimization: a survey. In: Waschl H, Kolmanovsky I, Steinbuch M, del Re L. (eds) Optimization and Optimal Control in Automotive Systems. Lecture Notes in Control and Information Sciences. Springer, Cham. 455:3–21
Balaji V, et al (2002) A graph neural network model for the prediction of longitudinal tau aggregation. J Nucl Med 63 (supplement 2) 2233
Weickenmeier J et al (2019) A physics-based model explains the prion-like features of neurodegeneration in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. J Mech Phys Solids 124:264–281
Meisl G et al (2021) In vivo rate-determining steps of tau seed accumulation in Alzheimer’s disease. Sci Adv 7(44):eabh1448
Lowe VJ et al (2018) Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141(1):271–287
Lozes F, Elmoataz A, Lézoray O (2014) Partial difference operators on weighted graphs for image processing on surfaces and point clouds. IEEE Trans Image Process 23(9):3896–3909
Elmoataz A, Lezoray O, Bougleux S (2008) Nonlocal discrete regularization on weighted graphs: a framework for image and manifold processing. IEEE Trans Image Process 17(7):1047–1060
Grigor’yan A (2018) Introduction to analysis on graphs, vol 71. American Mathematical Soc
Kondor RI, Lafferty J (2002) Diffusion kernels on graphs and other discrete structures. In: Proceedings of the 19th International Conference on Machine Learning
Zheng Y-Q et al (2019) Local vulnerability and global connectivity jointly shape neurodegenerative disease propagation. PLoS Biol 17(11):e3000495
Vogel JW et al (2020) Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun 11(1):2612
Iturria-Medina Y et al (2014) Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders. PLoS Comput Biol 10(11):e1003956
Desikan RS et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980
Cho H et al (2019) Progressive tau accumulation in Alzheimer disease: 2-year follow-up study. J Nucl Med 60(11):1611–1621
Kennan J (2006) A note on discrete approximations of continuous distributions. Univ. Wisconsin-Madison, Madison, WI, USA
Krogh A, Hertz J (1991) A simple weight decay can improve generalization. Adv Neural Inform Process Syst 4: 950–957
Kirkpatrick S, Gelatt CD Jr, Vecchi MP (1983) Optimization by simulated annealing. Science 220(4598):671–680
Tsallis C, Stariolo DA (1996) Generalized simulated annealing. Phys A 233(1–2):395–406
Xiang Y, Gong X (2000) Efficiency of generalized simulated annealing. Phys Rev E 62(3):4473
Nahar S, Sahni S, Shragowitz E (1986). Simulated annealing and combinatorial optimization. In: 23rd ACM/IEEE Design Automation Conference. 1986. IEEE
Obuchi T, Kabashima Y (2016) Sparse approximation problem: how rapid simulated annealing succeeds and fails. J Phys Conf Ser 699 012017
Stoer J, Bulirsch R (1991) Introduction to Numerical Analysis (Applied Mathematics vol 12). Springer, Berlin
Pan SJ, Yang Q (2010) A survey on transfer learning. IEEE Trans Knowl Data Eng 22(10):1345–1359
Hutter F, Hoos HH, Stützle T (2007) Automatic algorithm configuration based on local search. In: Aaai. 2007
Levenberg K (1944) A method for the solution of certain non-linear problems in least squares. Q Appl Math 2(2):164–168
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11(2):431–441
Lyness JN (1967) Numerical algorithms based on the theory of complex variable. In: Proceedings of the 1967 22nd National Conference. 1967
Ralston A (1962) Runge-Kutta methods with minimum error bounds. Math Comput 16(80):431–437
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):e17
He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17(10):2407–2419
Brin S, Page L (1998) The anatomy of a large-scale hypertextual web search engine. Comput Netw ISDN Syst 30(1–7):107–117
Rubinov M, Sporns O (2011) Weight-conserving characterization of complex functional brain networks. Neuroimage 56(4):2068–2079
Hawrylycz MJ et al (2012) An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489(7416):391–399
Cho H et al (2016) In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol 80(2):247–258
Insel PS et al (2020) Neuroanatomical spread of amyloid β and tau in Alzheimer’s disease: implications for primary prevention. Brain Commun 2(1):fcaa007
Thompson TB et al (2020) Protein-protein interactions in neurodegenerative diseases: a conspiracy theory. PLoS Comput Biol 16(10):e1008267
Van der Velden BH et al (2022) Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med Image Anal 79:102470
Berron D et al (2020) Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain 143(4):1233–1248
Harrison TM et al (2019) Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol 85(2):229–240
Leng F et al (2023) Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol Psychiatry 28(3):1303–1311
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124(1):1–38
Garcés P et al (2014) The Default Mode Network is functionally and structurally disrupted in amnestic mild cognitive impairment—A bimodal MEG–DTI study. NeuroImage Clin 6:214–221
Wu JW et al (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19(8):1085–1092
Yamada K et al (2014) Neuronal activity regulates extracellular tau in vivo. J Exp Med 211(3):387–393
Busche MA et al (2008) Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321(5896):1686–1689
Busche MA et al (2012) Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci 109(22):8740–8745
Castanho I et al (2020) Transcriptional signatures of tau and amyloid neuropathology. Cell Rep 30(6):2040-2054.e5
Zhou J et al (2012) Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73(6):1216–1227
Montal V et al (2022) Network Tau spreading is vulnerable to the expression gradients of APOE and glutamatergic-related genes. Sci Transl Med 14(655):eabn7273
Gong H et al (2013) Lipoprotein lipase (LPL) is associated with neurite pathology and its levels are markedly reduced in the dentate gyrus of Alzheimer’s disease brains. J Histochem Cytochem 61(12):857–868
Villavicencio Tejo F, Quintanilla RA (2021) Contribution of the Nrf2 pathway on oxidative damage and mitochondrial failure in Parkinson and Alzheimer’s disease. Antioxidants 10(7):1069
Caron F (2012) Bayesian nonparametric models for bipartite graphs. Adv Neural Inform Process Syst 25
Ni Y et al (2018) Bayesian graphical models for computational network biology. BMC Bioinforma 19(3):59–69
Matkowski J (2012) Mean-value theorem for vector-valued functions. Math Bohem 137(4):415–423
Jaynes ET (1957) Information theory and statistical mechanics. Phys Rev 106(4):620
Acknowledgements
This research was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (No. 2022R1A4A1033856), by the NRF grant funded by the MSIT (No. 2023R1A2C2006201, Development of simulation-based Digital-Brain editing technology), and by the Korea Disease Control and Prevention Agency (grant No. 2023-ER1003-01), funded by the Ministry of Health & Welfare, Republic of Korea. This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HU20C0164), and by a faculty research grant from Yonsei University College of Medicine (6-2022-0073).
Author information
Authors and Affiliations
Consortia
Contributions
J. K. Seong and C. H. Lyoo had full access to all the data in the study and took responsibility for the data's integrity and the data analysis's accuracy. S. W. Kim, H. Cho, J. K. Seong, and C. H. Lyoo planned the study design and concept. H. Cho and C. H. Lyoo collected image data. S. W. Kim and C. H. Lyoo preprocessed image data. S. W. Kim and Y. Lee performed analyses. S. W. Kim, H. Cho, C. H. Lyoo, and J. K. Seong interpreted the results and wrote the original manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file2 (AVI 50689 KB)
Supplementary file3 (AVI 49800 KB)
Supplementary file4 (AVI 26204 KB)
Supplementary file5 (AVI 90866 KB)
Supplementary file6 (AVI 88643 KB)
Appendices
Appendix
Appendix A. Derivation of the objective function for group-level optimization
We first note that the loss function \(\ell \left( {{{\varvec{\uptheta}}};\left\{ {\left( {t_{i,j} ,{\tilde{\mathbf{x}}}_{i,j} } \right)} \right\}_{j = 0}^{{m_{i} - 1}} ,H_{1} } \right)\) in the group-level optimization objective (3) quantifies the discrepancy as the difference between the average rate of change in observed tau topographic maps over \(\left[ {t_{i,0} ,t_{{i,m_{i} - 1}} } \right)\) and the expected instantaneous rates of changes evaluated by the tau spreading model. The average rate of change, denoted by \({\tilde{\mathbf{y}}}_{i}\), can be represented as follows:
\({\tilde{\mathbf{y}}}_{i} = \frac{{{\tilde{\mathbf{x}}}_{{i,m_{i} - 1}} - {\tilde{\mathbf{x}}}_{i,0} }}{{t_{{i,m_{i} - 1}} - t_{i,0} }} = \sum\limits_{j = 0}^{{m_{i} - 2}} {w_{i,j} \frac{{{\tilde{\mathbf{x}}}_{i,j + 1} - {\tilde{\mathbf{x}}}_{i,j} }}{{t_{i,j + 1} - t_{i,j} }}}\),
where \(w_{i,j} = \frac{{t_{i,j + 1} - t_{i,j} }}{{t_{{i,m_{i} - 1}} - t_{i,0} }}\) denotes the weight assigned to each sub-interval \(\left[ {t_{i,j} ,t_{i,j + 1} } \right)\). Let \({\dot{\mathbf{x}}}\left( {t;{{\varvec{\uptheta}}}} \right)\) denote the rate of instantaneous rates of changes evaluated by the tau spreading model \({\mathbf{x}}\left( {t;{{\varvec{\uptheta}}}} \right)\). By the mean-value theorem for vector-valued function [80], for a function differentiable \({\mathbf{x}}\left( t \right) = \left[ {\begin{array}{*{20}c} {x_{1} \left( t \right)} & {x_{2} \left( t \right)} & \ldots & {x_{k} \left( t \right)} \\ \end{array} } \right]^{{\text{T}}}\) over a bounded open interval, there exist constants \(t_{1} ,t_{2} ,...,t_{k}\) in the interval such that
with real numbers \(a\) and \(b\) in the interval. The exact values of the constants cannot be determined, however, so we tried to get the expectation of \({\dot{\mathbf{x}}}\left( {t;{{\varvec{\uptheta}}}} \right)\), rather than the raw value of \({\dot{\mathbf{x}}}\left( {t;{{\varvec{\uptheta}}}} \right)\), close to \({\tilde{\mathbf{y}}}_{i}\).
The expected value of \({\dot{\mathbf{x}}}\left( {t;{{\varvec{\uptheta}}}} \right)\) over the interval \(\left[ {t_{i,0} ,t_{{i,m_{i} - 1}} } \right)\), denoted by \({\hat{\mathbf{y}}}_{i}\), can be represented as follows:
where \(T_{i}\) denotes a random variable that can have a value between \(\left[ {t_{i,0} ,t_{{i,m_{i} - 1}} } \right)\) with the probability density function \(p\left( {T_{i} } \right)\). Since there is no information about the time constants except that they are in \(\left[ {t_{i,j} ,t_{i,j + 1} } \right)\), we approximate \(p\left( {T_{i} } \right)\) as a piecewise uniform distribution as follows:
according to the principle of maximum entropy [81]. Herein, \({\text{Uni}}\left( {t;t_{i,j} ,t_{i,j + 1} } \right)\) denotes a continuous uniform distribution with the time interval. The expected value still cannot be evaluated due to intractable integration; we divided each interval \(\left( {t_{i,j} ,t_{i,j + 1} } \right)\) into \(H_{1}\) sub-intervals and approximated the continuous uniform distribution for each interval to a discrete uniform distribution \({\text{Uni}}\left\{ {t;t_{i,j} ,t_{i,j + 1} } \right\}\) whose support contains midpoints of the \(H_{1}\) sub-intervals, according to the best discrete approximation having equally weighted support points [41]. The expected value is then computed as follows:
It is worth noting that for taking midpoints of the \(H_{1}\) sub-intervals, we divided each interval \(\left( {t_{i,j} ,t_{i,j + 1} } \right)\) into \(2H_{1}\) sub-intervals and took all odd-numbered points. Each value of \({\dot{\mathbf{x}}}\left( {\left\langle {\tau_{i,j}^{{\left( {2H_{1} } \right)}} } \right\rangle_{2k - 1} ;{{\varvec{\uptheta}}}} \right)\) was computed using the numerical simulation with the step size \(h_{1} = {{\left( {t_{i,j + 1} - t_{i,j} } \right)} \mathord{\left/ {\vphantom {{\left( {t_{i,j + 1} - t_{i,j} } \right)} {2H_{1} }}} \right. \kern-0pt} {2H_{1} }}\). Finally, the loss function for the group-level optimization problem was defined as the squared \(L_{2}\) norm of the average difference between \({\tilde{\mathbf{y}}}_{i}\) and \({\hat{\mathbf{y}}}_{i} \left( {{\varvec{\uptheta}}} \right)\), or equivalently formulated as follows:
Appendix B. Snapshot analysis
The snapshot analysis of tau tangle spreading encompassed two approaches: evaluating the relative importance of factors in the system and constructing vector fields to illustrate the proximal spreading patterns.
The relative influence of local reaction and diffusion is calculated for each node by dividing the absolute value of each term in Eq. (2) by their sum. Similarly, the relative influence of proximal and distal spreading on the diffusion of tau tangles was determined based on the decomposition \({\mathbf{L}} \cdot {\mathbf{x}}\left( t \right) = {\mathbf{L}}_{{{\text{prox}}}} \cdot {\mathbf{x}}\left( t \right) + {\mathbf{L}}_{{{\text{dist}}}} \cdot {\mathbf{x}}\left( t \right)\), where \({\mathbf{L}}_{{{\text{prox}}}}\) and \({\mathbf{L}}_{{{\text{dist}}}}\) represent weighted graph Laplacians of \({\mathbf{C}}_{{{\text{prox}}}}\) and \({\mathbf{C}}_{{{\text{dist}}}}\), respectively. The sign of the relative influence indicates whether tau SUVRs increase or decrease due to a specific term at that particular time.
The individualized vector field was constructed by assigning a gradient vector to each side of triangles within the pial surface mesh. For a triangle defined by vertices \(v_{1}\), \(v_{2}\), \(v_{3}\) and sides \(v_{1} v_{2}\), \(v_{2} v_{3}\), \(v_{3} v_{1}\), a gradient vector \(\kappa \cdot \sqrt {c_{{v_{1} v_{2} }} } \cdot \left( {\mathbf{x}_{{v_{1} }} \left( t \right) - \mathbf{x}_{{v_{2} }} \left( t \right)} \right) \cdot \left( {{\mathbf{v}}_{1} - {\mathbf{v}}_{2} } \right)\), where \({\mathbf{v}}_{1}\) and \({\mathbf{v}}_{2}\) represent the corresponding Cartesian coordinate points, was assigned to the side \(v_{1} v_{2}\). Similar gradient vectors were assigned to the sides \(v_{2} v_{3}\) and \(v_{3} v_{1}\). The representative vector of the triangle was determined as the Euclidean vector originating from its incenter and calculated as the average of the three gradient vectors of its sides.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, SW., Cho, H., Lee, Y. et al. Data-driven simulation of network-based tau spreading tailored to individual Alzheimer's patients. Engineering with Computers (2024). https://doi.org/10.1007/s00366-024-01988-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00366-024-01988-y