Abstract
Energy conservation associated with hibernation is maximized at the intersection of low body temperature (Tb), long torpor bouts, and few interbout arousals. In the arctic ground squirrel (Urocitellus parryii), energy conservation during hibernation is best achieved at ambient temperatures (Ta) around 0 °C; however, they spend the majority of hibernation at considerably lower Ta. Because arctic ground squirrels switch to mixed fuel metabolism, including protein catabolism, at extreme low Ta of hibernation, we sought to investigate how microbial urea-nitrogen recycling is used under different thermal conditions. Injecting squirrels with isotopically labeled urea (13C/15N) during hibernation at Ta’s of − 16 °C and 2 °C and while active and euthermic allowed us to assess the ureolytic activity of gut microbes and the amount of liberated nitrogen incorporated into tissues. We found greater incorporation of microbially-liberated nitrogen into tissues of hibernating squirrels. Although ureolytic activity appears higher in euthermic squirrels, liberated nitrogen likely makes up a smaller percentage of the available nitrogen pool in active, fed animals. Because non-lipid fuel is a limiting factor for torpor at lower Ta in this species, we hypothesized there would be greater incorporation of liberated nitrogen in animals hibernating at − 16 °C. However, we found higher microbial-ureolytic activity and incorporation of microbially-liberated nitrogen, particularly in the liver, in squirrels hibernating at 2 °C. Likely this is because squirrels hibernating at 2 °C had higher Tb and longer interbout arousals, a combination of factors creating more favorable conditions for gut microbes to thrive and maintain greater activity while giving the host more time to absorb microbial metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The evolution of endothermy uncoupled the mammalian lifestyle from thermal conditions, enabling nocturnal activity and niche expansion (Nespolo et al. 2011; Rezende and Bacigalupe 2015). Mammalian endothermy results from metabolic heat production and thus comes at an energetic cost (Nagy et al. 1999). In seasonal environments when conditions are energetically unfavorable, some mammals cope via temporary suspension of the high energy commitment of endothermic homeothermy, and instead become heterothermic endotherms and vacillate between episodes of deep torpor and arousal characteristic of hibernation (Heldmaier and Ruf 1992; Nowack et al. 2017).
Energy expenditure during torpor is typically 2–5% that of Basal Metabolic Rate (Heldmaier and Ruf 1992; Ratigan and McKay 2016). Although torpor during hibernation confers a dramatic energetic advantage that is crucial for survival (Nowack et al. 2017), there are associated physiological costs of torpor such as reduced immunocompetence (Bouma et al. 2013; Ratigan and McKay 2016), increased oxidative damage (Carey et al. 2003; Wei et al. 2018), sleep deprivation (Daan et al. 1991), telomere damage (Nowack et al. 2019), and tissue catabolism (Hindle et al. 2015; Lee et al. 2012). Moreover, the energetic savings of torpor during hibernation are reduced by the energetic costs of periodic interbout arousals (IBA) (Buck and Barnes 2000; Charlanne et al. 2022; Karpovich et al. 2009). Conserved energy associated with hibernation is maximized at the nexus of low Tb, long torpor bouts, and few IBAs (Buck and Barnes 2000; Heldmaier and Ruf 1992). For example, in the arctic ground squirrel (Urocitellus parryii), hibernation is most efficient at ambient temperatures (Ta) around 0 °C, where Tb is ~ 2 °C (Buck and Barnes 2000). When Ta decreases below 0 °C, torpor bouts shorten, arousals become more frequent, and arctic ground squirrels enter a sub-optimal energy-conserving state as metabolic rate (MR) increases proportionally with decreasing Ta and Tb (Buck and Barnes 2000; Ruf et al. 2022; Williams et al. 2011).
Arctic ground squirrels occupy some of the harshest habitats of the Arctic and subarctic and during winter hibernaculum temperatures average − 8.9 °C with minima reaching as low as − 25 °C in late winter (Buck and Barnes 1999b). Consequently, most of the hibernation season is spent in a sub-optimal energy-conserving state (Buck and Barnes 2000). Arctic ground squirrels have one of the longest hibernation seasons, lasting up to 9 months, during which neither food nor water is consumed (Buck and Barnes 1999b). They dramatically increase their fat stores during the active season in preparation for hibernation (Buck and Barnes 1999a; Sheriff et al. 2013), and rely largely on lipid metabolism during the long fast of hibernation (Richter et al. 2015). However, at hibernaculum temperatures below 0 °C, arctic ground squirrels subsidize lipid metabolism with catabolism of lean tissue to support thermogenesis, and the availability of non-lipid metabolic fuel has been hypothesized as a factor that limits torpor bout duration (Buck and Barnes 2000). Thus, energy savings come from entering a state of deep torpor; yet, in arctic ground squirrels, when hibernating at ambient temperatures below 0 °C, the use of mixed fuel metabolism risks depletion of non-lipid resources and jeopardizing body condition and future performance upon emergence from hibernation in the spring (McLean and Towns 1981; Watton and Keenleyside 1974). Changes brought to the Arctic due to global climate warming, such as changes to freeze–thaw cycles, snow cover duration and hibernaculum temperatures, hold the potential to affect hibernation energetics and thus body condition and future performance following hibernation (Chmura et al. 2023).
Arctic ground squirrels remodel specific tissues during hibernation, with protein synthesis occurring mostly in essential organs like the liver or heart (Bertile et al. 2021; Kurtz et al. 2021; Lee et al. 2012; Rice et al. 2020). In thirteen-lined ground squirrels, Hindle et al. (2015) presented evidence for reduced protein synthesis and skeletal muscle mass loss early in the hibernation season, followed by a shift to anabolism and muscle recovery in late winter. Similarly for the arctic ground squirrel, an increase in transcription of anabolic genes suggestive of protein biosynthesis during IBAs has been demonstrated (Goropashnaya et al. 2020). Altogether, the catabolism of lean mass interspersed with ongoing protein synthesis during hibernation, with prioritization patterns of different tissue type remodeling throughout stages of the hibernation season, indicates protein sparing mechanisms are available to the animals, enabling protein balance while fasting. A detailed review on protein sparing mechanisms in hibernation can be found in Bertile et al. (2021).
Recently Regan et al. (2022) demonstrated that microbial urea-nitrogen recycling (or urea nitrogen salvage; UNS) is a mechanism that facilitates protein synthesis in thirteen-lined ground squirrels, particularly in late hibernation. UNS is a process whereby urea, the product of amino acid degradation, is hydrolyzed in the host intestine into ammonia and carbon dioxide by ureolytic microbes. Liberated ammonia can be reabsorbed and used by the host, or it may be used by the resident microbes for synthetic processes, the end products of which can also be utilized by the host (Regan et al. 2022; Stewart and Smith 2005). The extent to which this process contributes to nitrogen balance varies not only among different species but also with respect to individual physiological state, protein demand, and dietary conditions (Regan et al. 2022; Stewart and Smith 2005).
Given that arctic ground squirrels switch to mixed fuel metabolism at low ambient temperatures of hibernation, and considering the potential impact of climate warming on hibernation conditions, we were particularly interested in investigating how microbial urea-nitrogen recycling is used under different hibernation conditions. We examined this in animals hibernating at two different ambient temperatures, − 16 °C and 2 °C, as well as in active euthermic squirrels. Briefly, hibernating and active animals were given a sequence of isotopically labeled urea injections (13C/15N), and subsequently measured for MRs and breath and tissue isotope values. Since non-lipid fuel is a limiting factor for torpor at lower Ta, we hypothesized we would find higher UNS markers in animals hibernating at − 16 °C compared to those hibernating at 2 °C. Because the summer active euthermic squirrels are feeding and have an abundance of dietary nitrogen available, we hypothesized that the incorporation of microbially liberated isotopes would be lower compared to the hibernating animals, despite a higher gut microbiome abundance and activity during the active season (Stevenson et al. 2014).
Material and methods
Experimental approach
In order to assess ureolytic activity in the gut and subsequent incorporation of microbially-liberated urea-nitrogen (MLUN) into tissues, we used an approach similar to that described by Harlow (Harlow 2012) and reported recently by Regan et al. (2022). In this approach, isotopically labeled urea 13C/15N/15N is injected intraperitoneally (IP), diffuses into the gut, and becomes available for hydrolysis by ureolytic microbes, releasing 15NH4 and 13CO2 which can be detected in tissues and breath, respectively. In our case, we not only introduce the urea to be salvaged, but compare the results of animals that received urea labeled with heavy isotopes to those that received unlabeled urea. We are able to follow the heavy isotopes within tissues because their introduction increases δ 15N and δ 13C values far above any changes due to metabolic fractionation. Additionally, because our experimental design (see below and Fig. 1) utilizes squirrels in “companion” treatments, whereby squirrels under the same conditions receive either labeled urea or unlabeled urea (control), all squirrels engage the same biochemical pathways, and any fractionation or differences in incorporation are accounted for by comparison. Consequently, observed increases in δ 13C and δ 15N ratios in experimental animals over control animals indicate incorporation of the labeled urea.
Breath sampling scheme in hibernating arctic ground squirrels. After at least 48 days of torpor an IBA was initiated (IBA1) by moving the squirrel to a warm room and administering the first urea injection, after which the squirrel was allowed to reenter torpor. A second IBA (IBA2) was initiated and injection administered, after which the squirrel was transferred to a metabolic chamber at the assigned hibernation temperature. When Tb reached 30 °C, MR was recorded and breath samples (3) collected over each of the following IBA, torpor bout, and subsequent IBA. Inset box shows number of animals injected with labeled (experimental) or unlabeled (control) urea at each hibernation temperature. Gray shading indicates when squirrels were in a metabolic chamber and MR was recorded and breath collected
Animals
We trapped juvenile free-living arctic ground squirrels (~ 50:50 M:F; Table S11) in the northern foothills of the Brooks Range, Alaska near the Atigun River (68° 38’ N; 149° 38’ W) in summer 2015 and 2016. Squirrels were transferred to the University of Alaska Anchorage animal holding facility and housed individually in hanging wire cages (48 × 31 × 31 cm) at a Ta = 19 °C (temperature within the thermal neutral zone of euthermic arctic ground squirrels) (Hock 1960) and 12:12 L:D. Squirrels were provided cotton bedding and metal tubes for enrichment, and rodent chow (Mazuri, Brentwood, MO, USA), and water ad libitum.
Squirrels were randomly assigned to one of three groups: (1) active season squirrels (n = 18), (2) squirrels hibernating at Ta = 2 °C (n = 11) and (3) squirrels hibernating at Ta = − 16 °C (n = 23). Due to a hibernation chamber malfunction, we had to exclude 13 squirrels in the − 16 °C group from the final analysis. Experimental manipulations (described below) of hibernating squirrels were initiated during their first hibernation season in captivity, whereas experimental manipulation of active season squirrels was initiated following one hibernation season in captivity.
Each squirrel received a sequence of IP injections (described below) of isotopically labeled urea (hereafter “experimental”; 50 mg/kg; 99% 13C, 98% 15N2, Cambridge Isotope Laboratories, Inc; MA, USA), or unlabeled urea (hereafter “control”; Thermo Fisher Scientific, Waltham, MA). After the final injection, squirrels were placed in an open flow respirometry system to quantify MR and collect breath samples for isotope analysis (13C; described below). After completion of measurements, animals were transferred to a surgery suite (21 °C ± 1 °C) and immediately euthanized by isoflurane overdose followed by decapitation (hibernating squirrels at the final euthermic hours of IBA3 and active euthermic squirrels after final 3rd breath sample collection). We collected samples of tissues reported to have varying degrees of remodeling during hibernation (heart, liver, small intestine, cecum and quadriceps muscle; Bertile et al. 2021; Kurtz et al. 2021; Lee et al. 2012; Rice et al. 2020). Tissues were collected, rinsed with sterile saline until the liquid ran clear, frozen, and stored (− 80 °C) for isotope analysis (13C/15N).
All procedures were approved by the UAA Institutional Animal Care and Use Committee (UAA IACUC protocols 769,548, 842,836).
Experimental design, injection and sampling scheme: hibernating squirrels
All squirrels to be manipulated during hibernation were surgically implanted with temperature sensitive transmitters (TA-F40; Data Sciences International, New Brighton, MN, USA) to monitor Tb and hibernation stage (torpor vs. IBA) in real time (Richter et al. 2015). For squirrels trapped in 2015, hibernation was monitored and hibernation stage logged from the time they entered hibernation (see below) through experimental manipulation and sampling using the DSI system. In 2016, hibernation was monitored and stage logged using the sawdust method (Pengelley and Fisher 1961) until just prior to experimental manipulation, at which time squirrels were transferred to DSI receiver boards and Tb was continuously monitored via DSI.
In the fall, all squirrels were transferred to an environmental chamber with conditions conducive to hibernation (Ta = 2 °C; L: D = 0:24; reduced food ration = ~ 25 g refreshed every 3 d as needed). Once animals entered torpor, we transferred them to Nalgene™ tubs (38 × 56 × 20.5 cm) containing wood shavings, cotton bedding and steel wire lids. For the remainder of hibernation, squirrels were held in constant darkness with neither food nor water. Squirrels assigned to hibernate at Ta = − 16 °C first entered torpor at Ta = 2 °C and were then transferred to the Ta = − 16 °C chamber during either their first or second IBA.
Squirrels assigned to hibernation Ta = − 16 °C spent on average 64.80 ± 3.43 days in the designated temperature, whereas for the squirrels from the Ta = 2 °C group the average was 115.40 ± 3.18 days. There were no differences between the experimental and control squirrels in the number of days they spent exposed to their respective hibernation temperatures (Ta = − 16 °C experimental, 64.15 ± 4.97; control, 65.42 ± 4.75. Ta = 2 °C experimental, 112.66 ± 3.75; control, 118.10 ± 5.12 days).
We administered urea injections at two sequential IBAs, and sampled tissues after the third IBA to enable sufficient time for MLUN to be incorporated into the squirrel’s tissue pool. After at least 48 days of cumulated torpor at their assigned temperature, we stimulated an IBA by moving the squirrel to a 19 °C dark room and administering the first urea injection, after which the squirrel was transferred back to the environmental chamber and allowed to complete the IBA and reenter torpor (Fig. 1). Then, after 48–72 h in torpor, a second IBA was stimulated and an injection administered, after which the squirrel was transferred to a metabolic chamber at the assigned hibernation temperature. The number of squirrels at each temperature receiving either labeled (experimental) or unlabeled (control) urea injections is depicted in Fig. 1. When Tb reached 30 °C (indicating the squirrel was in an IBA), MR was recorded continuously via open flow respirometry throughout the IBA (Fig. 1; see Respirometry details below), and breath samples collected every 3–4 h until Tb decreased below 30 °C (resulting in 3 or 4 breath collections, depending on the duration of the IBA). During the subsequent torpor bout, MR was recorded for approximately 12 h, and breath samples collected as described above (Fig. 1). Measurements were collected again as indicated during a third IBA (Fig. 1), after which the squirrel was euthanized, and tissue samples collected as described above.
Experimental design, injection and sampling scheme: active season squirrels
Squirrels assigned to the active season group were allowed to hibernate at Ta = 2 °C for the whole hibernation season. As described above, to promote hibernation squirrels were transferred to an environmental chamber maintained at 2 °C with reduced food rations and no light exposure. Once they entered torpor, we transferred them to plastic tubs containing wood shavings and cotton bedding. For the remainder of hibernation, squirrels were held in constant darkness with no food or water, and hibernation was monitored using the sawdust method (Pengelley and Fisher 1961). At the end of hibernation, squirrels were moved to a warm room (19 °C; 12:12 L:D), housed individually in hanging wire cages with cotton bedding, and provided rodent chow (Mazuri, Brentwood, MO, USA) and water ad libitum. At 4–6 weeks after ending heterothermy, squirrels were given two IP injections of labeled (n = 10) or unlabeled (n = 8) urea separated by 2 weeks. Immediately after the second injection, we began MR measurements for a total of nine hours and collected breath samples every three hours, after which squirrels were euthanized and tissue samples collected as described previously.
Analytical methods: respirometry
An open respirometry system was used to measure MR and respirometry exchange ratio (RER; average CO2 produced/O2 consumed) and collect excurrent air samples for 13C analysis of breath of three squirrels simultaneously. The respirometry system, including setup, calibration, software, and calculations, is described in detail in Tøien, (2013), Richter et al. (2015) and Lee et al. (2017). Briefly, squirrels were placed in hermetically sealed metabolic chambers (43 × 27 × 19 cm) at the ambient temperature of their assigned group. Ambient air was drawn from outside and through the metabolic chambers at a high rate 2.5 ± 0.02 l/min (measured with a Brooks 5851E mass flow controller; Coastal Instruments Inc. Burgaw, NC) with a subsample rate of 0.20 ± 0.002 l/min (measured with a Flowbar 8 mass flow meter; Sable Systems, Las Vegas, NV, USA), or at a low rate of 0.20 ± 0.002 l/min (measured with Flowbar 8). Flow rate was changed by computer-controlled baselining units (Sable Systems, Las Vegas, NV, USA) depending on animal MR as described in Lee et al. (2017), i.e. flow rate was high for animals during IBA or when summer active, and typically low during torpor. Excurrent air (analyzer flow = 0.1 l/min) was dried with Nafion dryers and analyzed for O2 (Oxzilla II, Sable Systems, Las Vegas, NV, USA) and CO2 concentration (CA-10A CO2, Sable Systems, Las Vegas, NV, USA; Lee et al. 2017). Data was collected, corrected for drift, and analyzed with LabGraph (see Tøien 2013). Squirrels were weighed (± 1 g; Ohaus Ranger RD-15LM, Parsippany, NJ, USA) prior to respirometry recordings.
Excurrent air samples for 13C analysis of breath were collected during MR recordings as in Lee et al. (2017). Air was withdrawn directly upstream of the analyzers via 4-way stopcocks and a needle into evacuated glass Exetainer vials (12 ml, Labco Limited, Buckinghamshire, UK). Samples were stored at room temperature until analysis.
Analytical methods: isotope analysis
To prepare tissues for isotope analysis, samples were thawed overnight, slowly freeze-dried over 48 h, and then pulverized into fine powder using a bead beater. Excurrent air samples and powdered tissue samples were analyzed at the Colorado Plateau Stable Isotope Laboratory at Northern Arizona University. Air samples were analyzed for δ13C values via continuous flow isotope ratio mass spectrometry using a GasBench II interfaced to a Delta Plus isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Tissue samples were analyzed for δ15N and δ13C values via continuous flow isotope ratio mass spectrometry using a Thermo-Electron Delta V Advantage isotope ratio mass spectrometer configured through a Finnigan CONFLO III (Thermo Fisher Scientific, Waltham, MA). The Carlo ERBA NC2100 elemental analyzer (Costech Scientific Inc., Valencia, CA) was used for the combustion and separation of C and N. All isotope values are expressed in delta notation as:
where X = the heavy isotope, R = the ratio of heavy to light isotope, the carbon standard was Vienna PeeDee Belemnite, and the nitrogen standard was atmospheric nitrogen. A basic mixing model was used to account for the CO2 present in the ambient air of the breath samples.
The fambient is the fraction of ambient air included in the sample and calculated as the concentration of CO2 of the ambient air in the empty chamber divided by the concentration of CO2 within the metabolic chamber containing the squirrel.
Statistics
Results for the hibernating squirrels were analyzed by means of repeated measures analysis of covariance (RM-ANCOVA) or ANCOVA with hibernation temperature (− 16 °C or 2 °C) and urea treatment (control vs. experimental) as fixed factors, measurement order (order of breath sample collection) as the repeated factor (for breath, MR, RER and Tb analysis), with appropriate covariates (MR, Tb, body mass [BM], IBA duration). When a significant interaction between the main effects was found, we broke down the analysis to compare the two hibernation groups and/or urea treatments separately. For active animals, we used RM-ANCOVA/ANCOVA with the urea treatment as the fixed factor, measurement order (RM-ANCOVA for breath, MR, RER analysis), and covariates (BM). We verified distribution of all data using the Shapiro–Wilk test.
All statistical analyses were performed with SAS 9.4 software (mixed procedure; SAS Institute, Cary, NC, USA).
Results
Hibernating squirrels
Metabolic characteristics of hibernating squirrels
Pre-hibernation BM did not differ between squirrels hibernating at 2 °C and those hibernating at − 16 °C, and there were no differences in pre-hibernation BM between squirrels assigned to the experimental versus control group at either Ta (Table S1, S2). Mean final BM at euthanasia was lower for squirrels hibernating at Ta = − 16 °C compared to those hibernating at Ta = 2 °C (Table S1, S2). Urea treatment (experimental vs. control) had no effect on the final BM (Table S1).
Metabolic rate (MR) sampled at the time of breath collection was higher in squirrels hibernating at − 16 °C compared to those hibernating at 2 °C (F1, 18 = 26.63; P < 0.001; Fig. 2), reflecting the higher thermoregulatory costs of low Ta exposure. Regardless of the ambient temperature of hibernation, MR was not affected by the urea treatment (experimental vs. control, F1, 18 = 0.01; P = 0.914). However, MR was affected by the order of measurement (F8, 94 = 45.68; P < 0.001) which reflected the subsequent IBAs and torpor bouts the squirrels were going through, with MR increasing during IBAs and decreasing during torpor in all squirrels (Fig. 2). We also found a significant hibernation temperature × urea treatment × measurement order interaction (F25, 94 = 2.18; P = 0.003). Analyzing MR separately for each temperature group showed that the urea treatment had indeed no effect (− 16 °C: F1, 8 = 1.80; P = 0.216; 2 °C: F1, 9 = 2.97; P = 0.119), but measurement order did have an effect (− 16 °C: F8, 36 = 21.33; P < 0.001; 2 °C: F8, 59 = 24.67; P < 0.001), and there were no interactions (− 16 °C: F8, 36 = 1.76, P = 0.117; 2 °C: F8, 59 = 0.74; P = 0.653). RER was not affected by either hibernation temperature (F1, 18 = 2.61; P = 0.123; Fig. 2) or urea treatment (F1, 18 = 2.42; P = 0.136; Table 1). RER, similar to MR, changed with the measurement order, and was slightly lower during torpor (F8, 94 = 2.12; P = 0.041; Fig. 2). There was no significant effect of the hibernation temperature × urea treatment × measurement order interaction on RER (F25, 94 = 0.96; P = 0.524).
Physiological characteristics of hibernation during torpor and IBA (shaded areas) measured three times during each event in squirrels from the experimental group given an isotopically labeled urea shot (black symbols) and control group given unlabeled urea (white symbols). Metabolic rate (A), RER (B) and Tb (C) in squirrels hibernating at − 16 °C and metabolic rate (D), RER (E) and Tb (F) in squirrels hibernating at 2 °C. Data presented as means ± s.e.m
Squirrel Tb recorded at the time of breath sampling differed between individuals hibernating at − 16 °C and 2 °C (F1, 18 = 155.92; P < 0.001; Fig. 2), with the latter being warmer during both IBA and torpor. We found no differences in Tb between the experimental and control squirrels (F1, 18 = 2.38; P = 0.140) but the measurement order showed a significantly influenced Tb (F9, 97 = 2890.49; P < 0.001), due to animals cycling through IBA and torpor bouts. Because of the significant hibernation temperature × urea treatment × measurement order interaction (F25, 97 = 8.53; P < 0.001), we analyzed Tb separately by hibernation temperature group and found that Tb was significantly affected by the urea treatment in squirrels hibernating at − 16 °C (F1, 8 = 6.26; P = 0.036), the order of measurement (F8, 39 = 903.33, P < 0.001), and found a significant interaction between the two factors (F8, 39 = 3.49; 0.003). Experimental squirrels hibernating at − 16 °C were on average slightly warmer compared to the controls during torpor (Fig. 2). Tb of squirrels hibernating at 2 °C was not affected by the urea treatment (F1, 9 = 2.58; P = 0.142) and we found only the previous effect of measurement order (F8, 59 = 2580.45; P < 0.001) and no interaction (F8, 59 = 0.36; F = 0.937).
IBA and torpor length
Squirrels hibernating at 2 °C had longer IBAs than squirrels hibernating at − 16 °C (IBA2: F1, 17 = 31.24, P < 0.001; IBA3: F1, 16 = 58.51, P < 0.001; Fig. 3). There was no difference in IBA duration between experimental and control squirrels regardless of hibernation temperature (IBA2: F1, 17 = 0.05, P = 0.818; IBA3: F1, 16 = 0.06, P = 0.809), nor did we find an effect of a hibernation temperature × urea treatment interaction (IBA2: F1, 17 = 0.07, P = 0.788; IBA3: F1, 16 = 0.62, P = 0.444). Similarly, torpor bout was longer in squirrels hibernating at 2 °C compared to − 16 °C (F1, 31 = 25.63, P < 0.001; Table 1). The urea treatment did not affect torpor bout length (F1, 32 = 1.54, P = 0.224), and there was no effect of a hibernation temperature × urea treatment interaction (F1, 31 = 1.23, P = 0.275) on torpor bout length.
Breath δ13C values in hibernating squirrels
To examine the ureolytic activity of gut microbiota in squirrels hibernating under different conditions, we collected breath samples as illustrated in Fig. 1. In our initial analysis, we found a significant three-way hibernation temperature × urea treatment × measurement order interaction (see Supplementary Material, Table S3); therefore, we analyzed breath δ13C values separately for each hibernation group (Table S4) and each urea treatment (Table S5). This revealed a significant effect of the urea treatment in squirrels hibernating at both ambient temperatures; experimental squirrels injected with labeled urea had higher δ13C breath values (Table S4, Fig. 4) compared to controls injected with unlabeled urea. There was also a significant effect of hibernation temperature in both experimental and control squirrels (Table S5) with those hibernating at 2 °C showing higher δ13C values in their breath across the entire measurement period (Fig. 4B) compared to those hibernating at − 16 °C. Interestingly, Tb affected the breath δ13C value only in the case of squirrels hibernating at 2 °C (Table S4). Measurement order (Table S4, S5) also significantly affected δ13C values in breath; it was higher during IBAs and lower during torpor (Fig. 4). Finally, we found a significant urea treatment × measurement order (Table S4) interaction and a hibernation temperature × measurement order (Table S5) interaction in the control group. These reflect the squirrels’ IBAs and torpor bout during the measurement period.
δ13C values in breath of hibernating squirrels. Breath was collected over two IBAs and the intervening torpor bout from experimental group given an isotopically labeled urea shot (black squares) and control group given unlabeled urea (white circles) hibernating at Ta − 16 °C (A) and 2 °C (B). Data presented as means ± s.e.m. Ns are shown in Fig. 1
Incorporation of microbially liberated 15N and 13C into hibernating squirrel tissue
The analysis of incorporation of microbially released 15N into squirrel tissues revealed a significant effect of the hibernation temperature and urea treatment (Table S7), with squirrels hibernating at 2 °C showing higher δ15N values, and the experimental group showing higher δ15N values regardless of the hibernation temperature in all analyzed tissue types. Of all the organs analyzed, liver tissue in all groups had significantly more 15N (Fig. 5). The quadriceps muscle δ15N value was significantly affected by the total duration of IBA during the experimental manipulation (Table S6). However, this analysis yielded, with the exception of the quadriceps muscle, a significant hibernation temperature × urea treatment interaction (Table S6). Thus, we analyzed the heart, liver, cecum and small intestine δ15N values separately for each hibernation temperature. This analysis showed that δ15N values in the cecum, intestine, liver and heart tissue were significantly affected by the urea treatment (Table S7), with experimental squirrels showing higher 15N incorporation (Fig. 5).
δ15N (A, C) and δ13C (B, D) values in tissue collected from hibernating squirrels. Samples were collected from the experimental group given an isotopically labeled urea shot (black symbols) and control group given unlabeled urea (white symbols) hibernating at − 16 °C (A, B) and 2 °C (C, D). Different letters show significant between-tissue differences (post hoc Tukey test). Data presented as means ± s.e.m. Ns are shown in Fig. 1
Contrary to δ15N results, δ13C values were the lowest in liver samples in both hibernating groups (Fig. 5). δ13C values were significantly affected by the hibernation temperature only in the heart, small intestine and quadriceps muscle samples (Table S8, Fig. 5) with squirrels hibernating at 2 °C incorporating more of the isotope. As expected, tissues of animals from the experimental group showed higher 13C incorporation (Table S8) in both hibernation temperatures (Fig. 5) compared to the control group, while IBA length affected only δ 13C values in the small intestine (Table S8). We found no significant interactions (Table S8).
Active season squirrels
Metabolic characteristics of active season squirrels
Active season squirrels were allowed to hibernate for one season in captivity prior to experimental manipulation, and BM prior to hibernation did not differ between those assigned to the experimental or control groups (F1, 52 = 0.28; P = 0.597; experimental: 772.05 ± 17.83 g; control: 786.25 ± 19.94 g). Similarly, the final BM recorded at the end of the experimental manipulation was not impacted by urea treatment (F1, 52 = 0.81; P = 0.372; experimental: 694.15 ± 17.65 g; control: 670.31 ± 19.74 g).
Metabolic rate recorded at the time of breath sample collection was not affected by either the urea treatment (F1, 16 = 0.06; P = 0.808) or the measurement order (F2, 32 = 0.70; P = 0.504; Table 1). There was no urea treatment × measurement order interaction (F2, 32 = 0.46; P = 0.637). Similarly, RER showed no effects of either the urea treatment (F1, 16 = 0.05; P = 0.816) or the measurement order (F2, 32 = 0.05; P = 0.949; Table 1), and there was no urea treatment × measurement order interaction (F2, 32 = 0.62; P = 0.542).
Breath δ13C values in active squirrels
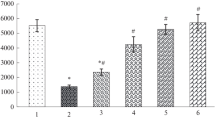
δ13C values in breath samples collected from the active euthermic squirrels were significantly affected by the urea treatment (F1, 12 = 173.58; P < 0.001; Fig. 6A); the experimental group showed higher δ13C values in their breath compared to controls. MR recorded at the time of breath collection had no effect on breath δ13C values (F1, 29 = 0.05; P = 0.832). Likewise, squirrel BM had no effect on breath δ13C values (F1, 14 = 0.01; P = 0.971). Measurement order had a significant effect (F2, 29 = 11.73; P = 0.001) with δ13C values decreasing over the course of the measurement period. However, because the urea treatment × measurement order interaction was significant (F2, 29 = 10.84; P = 0.001; Fig. 6A), we analyzed our data separately for each of the treatments and found a significant effect of the order of measurement in the experimental group, that showed that the labeled urea was indeed being metabolized over time (Table S9; Fig. 6A).
A Mean δ13C values in breath of active euthermic squirrels. Samples were collected across three timepoints from active euthermic squirrels from the experimental group given an isotopically labeled urea shot (n = 10; black symbols) and control group given unlabeled urea (n = 8; white symbols). δ15N values B and δ13C values C in tissue samples collected from active euthermic squirrels given labeled (experimental, n = 10; black symbols) and unlabeled (control, n = 8; white bars) urea injections. Different letters show significant between-tissue differences (post hoc Tukey test). Data presented as means ± s.e.m
Incorporation of microbially liberated 15N and 13C into tissue in active squirrels
Tissues collected from experimental squirrels injected with labeled urea were more enriched in 15N and 13C compared to those of control squirrels injected with unlabeled urea (Table S10, Fig. 6B, C). Squirrel BM had no effect on either 15N or 13C incorporation in the active season squirrels. The highest δ15N and δ 13C values in the active animals were recorded in the liver samples (Fig. 6B, C).
Discussion
Recently, the potential contribution of gut microbes to the maintenance of protein balance during hibernation was demonstrated by Regan et al. (2022), who found that hibernating 13-lined ground squirrels incorporate microbially-liberated urea nitrogen (MLUN) into their tissues. Our results confirm this in arctic ground squirrels, and here we show that the ambient temperature of hibernation affects both nitrogen recycling and host use of MLUN.
Although hibernation is a behavioral and physiological response enabling animals to cope with unfavorable environmental conditions, hibernaculum temperature (or Ta of hibernation) is a driver of the energetic costs associated with hibernation (Buck and Barnes 1999b). The hibernaculum temperature for arctic ground squirrels north of the Brooks Range in Arctic Alaska ranges from about − 5 to − 25 °C, invoking the need for constant heat production to maintain a Tb ≥ − 2.9 °C (Barnes 1989; Buck and Barnes 2000). As such, the gradient between Tb and Ta experienced by arctic ground squirrels, and against which they must defend Tb, is significantly larger than that of any known hibernator (Buck and Barnes 1999b), necessitating thermogenic increases and associated energetic costs. Ambient temperature-dependent patterns of MR and core Tb have been previously demonstrated over a range of temperatures (+ 20 to − 26 °C) in arctic ground squirrels (Buck and Barnes 2000; Richter et al. 2015). Our findings corroborate this, showing that squirrels hibernating at Ta = − 16 °C had a ten-fold higher MR during torpor, a lower core Tb, and lost more BM by the end of the experiment, than squirrels hibernating at Ta = 2 °C. We also observed differences in torpor bout and IBA length, reflecting the Ta-dependent costs associated with hibernation. Hibernation at high Ta (4–8 °C) results in more frequent arousals and elevated energy metabolism; however, the same applies to hibernation at low Ta (< 0 °C) where the Tb and Ta differential increases, eliciting higher metabolic heat production (Buck and Barnes 2000; Geiser and Kenagy 1988). Here, torpor bout length of squirrels hibernating at Ta = − 16 °C was approximately 1/3 the duration of squirrels hibernating at 2 °C, which is consistent with Buck and Barnes (Buck and Barnes 2000).
Hibernators rely predominantly on lipids to fuel their metabolism (Buck and Barnes 2000; Karpovich et al. 2009), but shift briefly to a combination of lipids and carbohydrates in the initial stages of rewarming during arousal (̴ 60 min) (Buck and Barnes 2000; Regan et al. 2019). In arctic ground squirrels, a similar shift to mixed fuel metabolism (RER ~ 0.80) occurred during steady state torpor at Ta below 0 °C (Buck and Barnes 2000), serving to fuel an increase in thermogenesis (Buck and Barnes 2000). Since ground squirrels are not gluconeogenic at low tissue temperatures characteristic of torpor (Galster and Morrison 1975), lean mass is catabolized during IBAs and stored as glycogen to replenish the gluconeogenic substrate pool (Galster and Morrison 1970; Krilowicz 1985). Our RER values for torpid squirrels at − 16 °C were around 0.7, which is indicative of lipid metabolism and in contrast to Buck and Barnes (Buck and Barnes 2000). However, we measured MR within the first ~ 12–24 h of a torpor bout, whereas Buck and Barnes (Buck and Barnes 2000) began their measurements 4 days after Tb of each squirrel fell below 30 °C. Neither study quantified body composition prior to metabolic trials, and since reliance on non-lipid fuels is influenced by endogenous lipid reserves (which are depleted later in the hibernation season), it is possible that the squirrels in Buck and Barnes had lower percent body fat at the time of their trials compared to ours. Additionally, unlike our study which included injections of urea and induced arousals, their experimental design did not include prior manipulation of the animals. Indeed, induced rather than natural arousals may have influenced metabolic fuel use in the intervening IBA (Utz and van Breukelen 2012), although precisely how is unknown. While Buck and Barnes (2000) manually manipulated the flow rate of the open respiratory system during torpor, we used an automated system and standard flow rate that may not have been sufficiently sensitive to capture the precise values. It is also worth noting that urea treatment and animal handling might have affected torpor bout duration, as we observed an average torpor length of approximately 3 days in squirrels hibernating at − 16 °C, compared to 7 days in Buck and Barnes (Buck and Barnes 2000). In addition, the experimental (injected with labeled urea) squirrels hibernating at Ta = − 16 °C had a higher Tb during torpor compared to controls injected with unlabeled urea, an effect that was absent in animals hibernating at 2 °C. This result is perplexing, as the urea dose was the same regardless of labeling, and urea treatment did not affect the MR of any of the squirrels.
The negative correlation between RER and maximum torpor length in Buck and Barnes (Buck and Barnes 2000) suggests that non-lipid fuel availability limits torpor duration at low Ta; thus, replenishing non-lipid fuels is critical for survival. Even though we could not detect the switch to mixed fuel use in our study, there are several reports showing at a molecular level that muscle breakdown occurs in arctic ground squirrels during hibernation and that nitrogen is recycled back into the protein pool (Fedorov et al. 2014; Goropashnaya et al. 2020; Rice et al. 2020). In fact, free-living female arctic ground squirrels have been shown to lose lean mass throughout winter (Buck and Barnes 1999a). Such catabolism of protein during hibernation at low Ta should necessitate additional urea synthesis in the liver, and increased urea transported to the gut should stimulate an increased ureolytic activity, resulting in a greater contribution of MLUN to the host in the form of NH3 (Fuller and Reeds 1998). While this may appear to contradict Rice et al. (2020), who demonstrated that urea cycle intermediates do not increase during torpor, they note an alternative explanation, namely that urea cycle intermediates may not accumulate in tissue due to microbial urease enzyme activity.
Injecting squirrels with isotopically labeled 13C/15N-urea allowed us to measure microbial ureolytic activity in vivo. Breath δ13C values increased in all experimental groups injected with labeled urea compared to controls injected with unlabeled urea, indicating microbial ureolysis occurred in hibernating and active squirrels. Overall, the highest δ13C breath values were recorded for euthermic active squirrels (Figs. 4, 6). This likely reflects a higher microbial abundance and activity in the guts of active versus hibernating arctic ground squirrels (Stevenson et al. 2014), and is consistent with Regan et al. (2022), who found higher breath δ13C values in summer euthermic compared to hibernating thirteen-lined ground squirrels.
We were also able to assess the incorporation of MLUN into tissues, and our results are consistent with Regan et al. (2022), who demonstrated a higher incorporation of 15N into tissue of hibernating compared to active season squirrels. We found that squirrels hibernating at 2 °C incorporated on average 150% more 15N in their tissues compared to squirrels hibernating at − 16 °C, and squirrels hibernating at − 16 °C incorporated on average 60% more than active season squirrels. The length of time in hibernation prior to sampling may have contributed to our results, as squirrels hibernating at 2 °C were sampled later in their hibernation season than those at − 16 °C, and it has been demonstrated that protein anabolism increases toward late hibernation (Hindle et al. 2015). Despite that active season arctic ground squirrels have an order of magnitude higher density of microbes (and potentially more ureolytic microbes) in their gut compared to hibernating individuals (Stevenson et al. 2014), perhaps this is not surprising given that captive active season squirrels should not need to rely on MLUN to meet their nitrogen demands. Even with high rates of ureolysis indicated by breath δ13C values in active season individuals, incorporation of MLUN might be lower in active squirrels than anticipated, as liberated urea-nitrogen should represent only a small fraction of the nitrogen available to fed squirrels. Although our active season squirrels had hibernated one season in captivity prior to the experimental manipulations, we do not anticipate that the hibernation conditions prior to the active season had an impact on our results, given that the gut microbiota returns to pre-hibernation diversity and composition within approximately 2 weeks of ending hibernation (Carey et al. 2013). In their study of thirteen-lined ground squirrels, Regan et al. (2022) investigated the pattern of urea-N recycling over the hibernation season and found the highest incorporation of 15N into tissue in late hibernation, which coincided with the lowest ureolytic activity (as determined by breath δ13C). However, they also showed the abundance of urea transporters in cecal tissue was higher in late winter compared to summer (Regan et al. 2022), suggesting the capacity for urea transport increases throughout the hibernation and is also matched by a shift to a more ureolytic microbiome as winter progresses (Regan et al. 2022). We cannot rule out the possibility that some unhydrolyzed (labeled) urea remained in our tissue samples, potentially influencing our results. However, we saw differing patterns of δ13C versus δ15N enrichment of tissues, and no differences in the δ13C of tissues from experimental (labeled-urea injected) compared to control animals hibernating at 2 °C, despite that both 13C and 15N were measured in the same samples. Additionally, our δ13C breath data suggests that microbial hydrolysis of urea occurs as early as 3 h after urea injections. These results are similar to those of Regan et al. (2022), who also found rapid urea hydrolysis. In their study, a significantly higher dose of urea was used (200 vs. 50 mg/kg) and they harvested tissues sooner after injection than in this study. Given this, we are confident that our results reflect the incorporation of MLUN into tissues.
We did not determine the pathways or the form by which MLUN was incorporated into tissues. However, it is likely that MLUN is incorporated into the protein pool as amino acids (Bertile et al. 2021; Rice et al. 2020). In their study of ammonia recycling during hibernation, Rice et al. (2020) proposed pathways by which free 15N-ammonia may be recycled into amino acids, predominantly during arousals. Additionally, other studies in non-hibernating species show that microbially-produced amino acids may be incorporated into the host protein pool (Besser et al. 2023). In a separate study, we have generated preliminary data suggesting that MLUN may be incorporated into amino acids such as citrulline (unpublished data), which are known to influence protein balance and trans-organ nitrogen balance (Moinard and Cynober 2007).
One might expect squirrels with higher MRs to have a higher incorporation of 13C/15N into their tissues, in which case we would expect to see higher δ15N values in tissues of arctic ground squirrels hibernating at − 16 °C compared to those at 2 °C; however, between the two hibernating temperatures we studied, more 15N and 13C was incorporated into tissues of squirrels hibernating at 2 °C. Even though counterintuitive, similar results have been obtained in other species. In house sparrows for example, cold-induced high MR did not result in an increased incorporation of 13C or 15N into tissue (Carleton and del Rio 2005). In addition, Voigt et al. (2003) found 13C incorporation in nectar-feeding bats to be one of the lowest recorded among vertebrates despite their high MRs. It seems that the effect of MR on tissue isotope incorporation is indirect and likely mediated by protein turnover (Carleton and del Rio 2005).
Our study was designed to investigate the use of microbially liberated urea-13C/15N in hibernating as well as active season animals; thus, the complicated interplay between microbiome activity and host physiology must be considered when discussing isotope incorporation. Compared to an IBA, host metabolic activity is lower during torpor. During arousals, when Tb returns to euthermia, there is an increase in both host metabolism (Karpovich et al. 2009) as well as microbial activity (Stevenson et al. 2014). Ground squirrels not only catabolize lean mass during arousals (Lee et al. 2012), but there is evidence of protein anabolism (Fedorov et al. 2014; Goropashnaya et al. 2020; Lee et al. 2012; Rice et al. 2020). Rice et al. (2020) showed that compared to kidney, liver, or lung tissue, 15N incorporation is lowest in skeletal muscle, leading to the speculation that this is caused by the lag in blood flow to the extremities during arousal and the sequence of body region rewarming (the head and thoracic region rewarms faster (Regan et al. 2019)). Squirrels in our study showed the same pattern of 15N incorporation at both hibernation temperatures, with the highest 15N incorporation in liver tissue, and the lowest (albeit not significantly different from the cecum, intestine and heart) in the quadriceps muscle. Thirteen-lined ground squirrels also showed the lowest isotope incorporation into muscle and highest in the liver during hibernation (Regan et al. 2022). Rice et al. (2020) also found that 15N incorporation was temperature-dependent, indicating that if monitored throughout full arousal, the recycling rate should be higher. We found the IBA duration affected 15N incorporation in the quadriceps muscle and 13C in the small intestine, which is somewhat consistent with the proposed explanation. Since ureolysis is temperature-dependent (Mitchell and Ferris 2005), one can speculate that rates of ureolysis should be higher in squirrels hibernating at 2 °C compared to − 16 °C. Squirrels hibernating at 2 °C not only maintain a higher Tb during torpor compared to − 16 °C, but their IBAs are longer (emaintaining euthermia for longer), providing more time for microbial ureolytic activity and higher rates of urea-13C/15N incorporation. This coincides with the thermal conditions at which the most energy conserving benefits of hibernation are achieved.
Our results demonstrate the complexity between urea hydrolysis and incorporation of MLUN into tissues under different hibernation conditions; however, they do not support our original hypothesis. We assumed since non-lipid fuel is a limiting factor for torpor at lower Ta, we would find higher UNS markers in animals hibernating at − 16 °C. Aside from the hibernators in the Arctic, such as the arctic ground squirrel and the Alaska Marmot (Marmota browerii; (Lee et al. 2009, 2016)), it is unlikely that other hibernators naturally experience extreme low hibernaculum temperatures and therefore spend most of the time close to the ideal hibernation temperature. Then again, the thirteen-lined ground squirrel study demonstrated that most benefits from the microbial urea-N recycling are observed in late hibernation (Regan et al. 2022), which might be concurrent with milder thermal conditions towards the end of the hibernation season. That study was, however, carried out in a controlled environment at a steady temperature of 4 °C; therefore, it would be interesting to investigate UNS patterns further not only throughout the hibernation season, but also over thermal gradients to which animals are naturally exposed. This would contribute to understanding and predicting how individuals and populations respond to rapidly changing climate conditions, particularly in the Arctic (Chmura et al. 2023). For example, over the last two decades alone global climate change has affected freeze–thaw cycles and snow cover duration in the Arctic (Chmura et al. 2023), leading to phenological shifts in some populations of arctic ground squirrels that might cause a mismatch between the sexes' receptiveness to reproduction (Williams et al. 2017). On the other hand, such environmental changes will certainly affect hibernation energetics, both reducing the time in hibernation as well as energy expenditure in warmer conditions. Our results suggest that hibernation at warmer ambient temperatures may provide for the added benefit of higher urea-N incorporation during the winter fast, which has the potential to increase overwinter survival (Sheriff et al. 2017; Chmura et al. 2023).
Data availability
Data supporting the findings of this study are available as supplementary materials.
References
Barnes BM (1989) Freeze avoidance in a mammal: body temperatures below 0 °C in an arctic hibernator. Science 244(4912):1593–1595. https://doi.org/10.1126/science.2740905
Bertile F, Habold C, Le Maho Y, Giroud S (2021) Body protein sparing in hibernators: a source for biomedical innovation. Front Physiol 12:634953. https://doi.org/10.3389/fphys.2021.634953
Besser AC, Manlick PJ, Blevins CM, Takacs-Vesbach CD, Newsome SD (2023) Variation in gut microbial contribution of essential amino acids to host protein metabolism in a wild small mammal community. Ecol Lett 26(8):1359–1369. https://doi.org/10.1111/ele.14246
Bouma HR, Henning RH, Kroese FGM, Carey HV (2013) Hibernation is associated with depression of T-cell independent humoral immune responses in the 13-lined ground squirrel. Dev Comp Immunol 39(3):154–160. https://doi.org/10.1016/j.dci.2012.11.004
Buck CL, Barnes BM (1999a) Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J Mammal 80(2):430–442. https://doi.org/10.2307/1383291
Buck CL, Barnes BM (1999b) Temperatures of hibernacula and changes in body composition of arctic ground squirrels over winter. J Mammal 80(4):1264–1276. https://doi.org/10.2307/1383177
Buck CL, Barnes BM (2000) Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol 279(1):R255–R262. https://doi.org/10.1152/ajpregu.2000.279.1.R255
Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83(4):1153–1181. https://doi.org/10.1152/physrev.00008.2003
Carey HV, Walters WA, Knight R (2013) Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. Am J Physiol Regul Integr Comp Physiol 304(1):R33–R42. https://doi.org/10.1152/ajpregu.00387.2012
Carleton SA, del Rio CM (2005) The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia 144(2):226–232. https://doi.org/10.1007/s00442-005-0066-8
Charlanne LM, Vetter S, Einwaller J, Painer J, Gilbert C, Giroud S (2022) Sticking together: energetic consequences of huddling behavior in hibernating juvenile garden dormice. Physiol Biochem Zool 95(5):400–415. https://doi.org/10.1086/721184
Chmura HE, Duncan C, Burrell G, Barnes BM, Buck CL, Williams CT (2023) Climate change is altering the physiology and phenology of an arctic hibernator. Science 380(6647):846–849. https://doi.org/10.1126/science.adf5341
Daan S, Barnes BM, Strijkstra AM (1991) Warming up for sleep?—Ground squirrels sleep during arousals from hibernation. Neurosci Lett 128(2):265–268. https://doi.org/10.1016/0304-3940(91)90276-Y
Fedorov VB, Goropashnaya AV, Stewart NC, Tøien Ø, Chang C, Wang H, Yan J, Showe LC, Showe MK, Barnes BM (2014) Comparative functional genomics of adaptation to muscular disuse in hibernating mammals. Mol Ecol 23(22):5524–5537. https://doi.org/10.1111/mec.12963
Fuller MF, Reeds PJ (1998) Nitrogen recycling in the gut. Annu Rev Nutr 18:385–411
Galster WA, Morrison PR (1970) Cyclic changes in carbohydrate concentrations during hibernation in the arctic ground squirrel. Am J Physiol Legac Content 218:1228–1232
Galster WA, Morrison PR (1975) Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol Legac Content 228:325–330
Geiser F, Kenagy GJ (1988) Torpor duration in relation to temperature and metabolism in hibernating ground squirrels. Physiol Zool 61(5):442–449. https://doi.org/10.1086/physzool.61.5.30161266
Goropashnaya AV, Barnes BM, Fedorov VB (2020) Transcriptional changes in muscle of hibernating arctic ground squirrels (Urocitellus parryii): implications for attenuation of disuse muscle atrophy. Sci Rep 10(1):9010. https://doi.org/10.1038/s41598-020-66030-9
Harlow H (2012) Muscle protein and strength retention by bears during winter fasting and starvation. In: McCue MD (Ed.), Comparative Physiology of Fasting, Starvation, and Food Limitation (pp. 277–296). Springer. https://link.springer.com/10.1007/
Heldmaier G, Ruf T (1992) Body temperature and metabolic rate during natural hypothermia in endotherms. J Comp Physiol B 162(8):696–706. https://doi.org/10.1007/BF00301619
Hindle AG, Otis JP, Epperson LE, Hornberger TA, Goodman CA, Carey HV, Martin SL (2015) Prioritization of skeletal muscle growth for emergence from hibernation. J Exp Biol. https://doi.org/10.1242/jeb.109512
Hock RJ (1960) Seasonal variation on physiologic functions of arctic ground squirrels and black bears. Bull Mus Comp Zool 124:155–173
Karpovich SA, Tøien Ø, Buck CL, Barnes BM (2009) Energetics of arousal episodes in hibernating arctic ground squirrels. J Comp Physiol B 179(6):691–700. https://doi.org/10.1007/s00360-009-0350-8
Krilowicz BL (1985) Ketone body metabolism in a ground squirrel during hibernation and fasting. Am J Physiol Regul Integr Comp Physiol 249:R462–R470
Kurtz CC, Otis JP, Regan MD, Carey HV (2021) How the gut and liver hibernate. Comp Biochem Physiol a: Mol Integr Physiol 253:110875. https://doi.org/10.1016/j.cbpa.2020.110875
Lee TN, Barnes BM, Buck CL (2009) Body Temperature Patterns during Hibernation in a Free-Living Alaska Marmot (Marmota Broweri). Ethol Ecol Evol 21(3–4):403–413. https://doi.org/10.1080/08927014.2009.9522495
Lee TN, Buck CL, Barnes BM, O’Brien DM (2012) A test of alternate models for increased tissue nitrogen isotope ratios during fasting in hibernating arctic ground squirrels. J Exp Biol. https://doi.org/10.1242/jeb.068528
Lee TN, Kohl F, Buck CL, Barnes BM (2016) Hibernation strategies and patterns in sympatric arctic species, the Alaska marmot and the arctic ground squirrel. J Mammal 97(1):135–144. https://doi.org/10.1093/jmammal/gyv163
Lee TN, Richter MM, Williams CT, Tøien Ø, Barnes BM, O’Brien DM, Buck CL (2017) Stable isotope analysis of CO2 in breath indicates metabolic fuel shifts in torpid arctic ground squirrels. Comp Biochem Physiol a: Mol Integr Physiol 209:10–15. https://doi.org/10.1016/j.cbpa.2017.04.004
McLean IG, Towns AJ (1981) Differences in weight changes and the annual cycle of male and female arctic ground squirrels. Arctic 34(3):249–254. https://doi.org/10.14430/arctic2527
Mitchell AC, Ferris FG (2005) The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: temperature and kinetic dependence. Geochim Cosmochim Acta 69(17):4199–4210. https://doi.org/10.1016/j.gca.2005.03.014
Moinard C, Cynober. (2007) Citrulline: a new player in the control of nitrogen homeostasis. J Nutr 137(6 Suppl 2):1621S-1625S
Nagy KA, Girard IA, Brown TK (1999) Energetics of free-ranging mammals, reptiles And BIRDS. Annu Rev Nutr 19(1):247–277. https://doi.org/10.1146/annurev.nutr.19.1.247
Nespolo RF, Bacigalupe LD, Figueroa CC, Koteja P, Opazo JC (2011) Using new tools to solve an old problem: the evolution of endothermy in vertebrates. Trends Ecol Evol 26(8):414–423. https://doi.org/10.1016/j.tree.2011.04.004
Nowack J, Stawski C, Geiser F (2017) More functions of torpor and their roles in a changing world. J Comp Physiol B 187(5–6):889–897. https://doi.org/10.1007/s00360-017-1100-y
Nowack J, Tarmann I, Hoelzl F, Smith S, Giroud S, Ruf T (2019) Always a price to pay: hibernation at low temperatures comes with a trade-off between energy savings and telomere damage. Biol Let 15(10):20190466. https://doi.org/10.1098/rsbl.2019.0466
Pengelley E, Fisher K (1961) Rhythmical arousal from hibernation in the golden-mantled ground squirrel, Citellus lateralis tescorum. Can J Zool 39:105–120
Ratigan ED, McKay DB (2016) Exploring principles of hibernation for organ preservation. Transplant Rev 30(1):13–19. https://doi.org/10.1016/j.trre.2015.08.002
Regan MD, Chiang E, Martin SL, Porter WP, Assadi-Porter FM, Carey HV (2019) Shifts in metabolic fuel use coincide with maximal rates of ventilation and body surface rewarming in an arousing hibernator. Am J Physiol Regul Integr Comp Physiol 316(6):R764–R775. https://doi.org/10.1152/ajpregu.00379.2018
Regan MD, Chiang E, Liu Y, Tonelli M, Verdoorn KM, Gugel SR, Suen G, Carey HV, Assadi-Porter FM (2022) Nitrogen recycling via gut symbionts increases in ground squirrels over the hibernation season. Science 375(6579):460–463. https://doi.org/10.1126/science.abh2950
Rezende EL, Bacigalupe LD (2015) Thermoregulation in endotherms: physiological principles and ecological consequences. J Comp Physiol B 185(7):709–727. https://doi.org/10.1007/s00360-015-0909-5
Rice SA, Ten Have GAM, Reisz JA, Gehrke S, Stefanoni D, Frare C, Barati Z, Coker RH, D’Alessandro A, Deutz NEP, Drew KL (2020) Nitrogen recycling buffers against ammonia toxicity from skeletal muscle breakdown in hibernating arctic ground squirrels. Nat Metab 2(12):1459–1471. https://doi.org/10.1038/s42255-020-00312-4
Richter MM, Williams CT, Lee TN, Tøien Ø, Florant GL, Barnes BM, Buck CL (2015) Thermogenic capacity at subzero temperatures: How low can a hibernator go? Physiol Biochem Zool 88(1):81–89. https://doi.org/10.1086/679591
Ruf T, Giroud S, Geiser F (2022) Hypothesis and theory: a two-process model of torpor-arousal regulation in hibernators. Front Physiol 13:901270. https://doi.org/10.3389/fphys.2022.901270
Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL (2013) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86(5):515–527. https://doi.org/10.1086/673092
Sheriff MJ, Boonstra R, Palme R, Buck CL, Barnes BM (2017) Coping with differences in snow cover: the impact on the condition, physiology and fitness of an arctic hibernator. Conserv Physiol 5(1):cox065. https://doi.org/10.1093/conphys/cox065
Stevenson TJ, Duddleston KN, Buck CL (2014) Effects of season and host physiological state on the diversity, density, and activity of the arctic ground squirrel cecal microbiota. Appl Environ Microbiol 80(18):5611–5622. https://doi.org/10.1128/AEM.01537-14
Stewart GS, Smith CP (2005) Urea nitrogen salvage mechanisms and their relevance to ruminants, non-ruminants and man. Nutr Res Rev 18(1):49–62. https://doi.org/10.1079/NRR200498
Tøien Ø (2013) Automated open flow respirometry in continuous and long-term measurements: design and principles. J Appl Physiol 114(8):1094–1107. https://doi.org/10.1152/japplphysiol.01494.2012
Utz JC, van Breukelen F (2012) Prematurely induced arousal from hibernation alters key aspects of warming in golden-mantled ground squirrels. Callospermophilus Lateralis 8(38):570–575. https://doi.org/10.1016/j.jtherbio.2013.10.001
Voigt CC, Matt F, Michener R, Kunz TH (2003) Low turnover rates of carbon isotopes in tissues of two nectar-feeding bat species. J Exp Biol 206(8):1419–1427. https://doi.org/10.1242/jeb.00274
Watton DG, Keenleyside MHA (1974) Social behaviour of the arctic ground squirrel. Spermophil Us und Ulat Us Behaviour 50(1–2):77–99. https://doi.org/10.1163/156853974X00048
Wei Y, Zhang J, Xu S, Peng X, Yan X, Li X, Wang H, Chang H, Gao Y (2018) Controllable oxidative stress and tissue specificity in major tissues during the torpor–arousal cycle in hibernating Daurian ground squirrels. Open Biol 8(10):180068. https://doi.org/10.1098/rsob.180068
Williams CT, Goropashnaya AV, Buck CL, Fedorov VB, Kohl F, Lee TN, Barnes BM (2011) Hibernating above the permafrost: effects of ambient temperature and season on expression of metabolic genes in liver and brown adipose tissue of arctic ground squirrels. J Exp Biol 214(8):1300–1306. https://doi.org/10.1242/jeb.052159
Williams CT, Buck CL, Sheriff MJ, Richter MM, Krause JS, Barnes BM (2017) Sex-dependent phenological plasticity in an arctic hibernator. Am Natur. https://doi.org/10.1086/694320
Acknowledgements
We thank our squirrel team Jasmine Hatton, Sarah Gering, and Shannon Medlock for help with animal husbandry and lab work.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (1R15DK110784) and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institute of Health (P20GM103395). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH. Support for KMC also provided by the Alaska Native Science and Engineering Society and the Sloan Scholar, Alfred P. Sloan Foundation’s Indigenous Graduate Partnership Program (SIGP). JS was supported by the Bekker Programme, NAWA.
Author information
Authors and Affiliations
Contributions
KND, CLB developed the study concept and experimental design and KND oversaw all aspects of the work from inception to manuscript preparation. KMC carried out the experiment and collected the data. JS analyzed the data and drafted the original manuscript and figures. TNL assisted with isotope data collection and analysis. KND, KMC, TNL and CLB provided critical revisions.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no conflict of interest.
Additional information
Communicated by Kathrin H Dausmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Julita Sadowska and Karen M. Carlson are first co-authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sadowska, J., Carlson, K.M., Buck, C.L. et al. Microbial urea-nitrogen recycling in arctic ground squirrels: the effect of ambient temperature of hibernation. J Comp Physiol B (2024). https://doi.org/10.1007/s00360-024-01579-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00360-024-01579-9