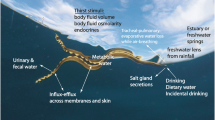
Abstract
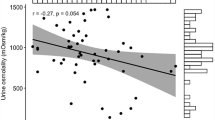
Hagfish are osmoconformers, maintaining an internal osmolality that matches their seawater habitats. Hagfish would, therefore, appear to have no physiological need to drink, but previous studies are equivocal regarding whether drinking in hagfish occurs. The current study addressed this knowledge gap, by examining drinking and water permeability in the Pacific hagfish, Eptatretus stoutii. One-third of analysed hagfish were shown to accumulate radiolabelled drinking rate markers (tritiated inulin and polyethylene glycol-4000) in their gut tissues; however, this was attributed to the presence of markers in the blood perfusing the digestive tract, following absorption through paracellular pathways at the gill. No accumulation of marker was observed in hagfish subjected to more dilute (75% seawater) or more concentrated (125% seawater) media. Diffusive water efflux, measured by tritiated water washout, was shown to be very high, with 50% of body water exchanged within 14 to 16 min, depending on exposure salinity. In full-strength seawater, the total exchangeable pool of water was 78% of hagfish mass. We conclude that hagfish do not drink, and their high water permeability is likely to result in rapid osmotic equilibration under circumstances where perturbations may occur.




Similar content being viewed by others
References
Alt JM, Stolte H, Eisenbach GM, Walvig F (1981) Renal electrolyte and fluid excretion in the Atlantic hagfish Myxine glutinosa. J Exp Biol 91:323–330
Armbruster DA, Pry T (2008) Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 29:S49–S52.
Bellamy D, Chester-Jones I (1961) Studies on Myxine glutinosa-1. The chemical composition of the tissues. Comp Biochem Physiol 3:175–183
Best JH, Eddy FB, Codd GA (2003) Effects of Microcystis cells, cell extracts and lipopolysaccharide on drinking and liver function in rainbow trout Oncorhynchus mykiss Walbaum. Aquat Toxicol 64:419–426
Beyenbach KW, Kirschner LB (1976) The unreliability of mammalian glomerular markers in teleostean renal studies. J Exp Biol 64:369–378
Cholette C, Gagnon A, Germain P (1970) Isosmotic adaptation in Myxine glutinosa L. 1. Variations of some parameters and role of the amino acid pool of the muscle cells. Comp Biochem Physiol 33:333–346
Clifford AM, Guffey SC, Goss GG (2014) Extrabranchial mechanisms of systemic pH recovery in hagfish (Eptatretus stoutii). Comp Biochem Physiol A 168:82–89
Clifford AM, Goss GG, Wilkie MP (2015) Adaptations for a deep sea scavenger: extreme ammonia tolerance and active NH4 + excretion by the Pacific hagfish (Eptatretus stoutii). Comp Biochem Physiol A 182:64–74
Dall W, Smith DM (1977) Measurement of water drinking rate in marine crustaceans. J Exp Mar Biol Ecol 30:199–208
Evans DH (1968) Measurement of drinking rates in fish. Comp Biochem Physiol 25:751–753
Evans DH (1969) Studies on the permeability to water of selected marine, freshwater and euryhaline teleosts. J Exp Biol 50:689–703
Forster ME (1990) Confirmation of the low metabolic rate of hagfish. Comp Biochem Physiol A 96:113–116
Forster ME, Davison W, Satchell GH, Taylor HH (1989) The subcutaneous sinus of the hagfish Eptatretus cirrhatus and its relation to the central circulating blood volume. Comp Biochem Physiol A 93:607–612
Forster ME, Russell MJ, Hambleton DC, Olson KR (2001) Blood and extracellular fluid volume in whole body and tissues of the Pacific hagfish, Eptatretus stouti. Physiol Biochem Zool 74:750–756
Ghandehari H, Smith PL, Ellens H, Yeh PY, Kopecek J (1997) Size-dependent permeability of hydrophilic probes across rabbit colonic epithelium. J Pharmacol Exp Therapeut 280:747–753
Glover CN, Bucking C (2016) Feeding, digestion and nutrient absorption in hagfish. In: Edwards SL, Goss GG (eds) Hagfish Biology. CRC Press, Boca Raton, FL, pp 287–308
Glover CN, Bucking C, Wood CM (2011) Adaptations to in situ feeding: novel nutrient acquisition pathways in an ancient vertebrate. Proc Roy Soc B 278:3096–3101.
Glover CN, Bucking C, Wood CM (2013) The skin of fish as a transport epithelium: a review. J Comp Physiol B 183:877–891
Glover CN, Blewett TA, Wood CM (2015) Novel route of toxicant exposure in an ancient extant vertebrate: nickel uptake by hagfish skin and the modifying effects of slime. Environ Sci Technol 49:1896–1902
Glover CN, Niyogi S, Blewett TA, Wood CM (2016) Iron transport across the skin and gut epithelia of Pacific hagfish: kinetic characterisation and effect of hypoxia. Comp Biochem Physiol A 199:1–7
Glover CN, Blewett TA, Wood CM (2017) Effect of environmental salinity manipulation on uptake rates and distribution patterns of waterborne amino acids in the Pacific hagfish. Comp Biochem Physiol A 204:164–168
Grosell M, McDonald MD, Walsh PJ, Wood CM (2004) Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta). II: copper accumulation, drinking rate and Na+/K+-ATPase activity in osmoregulatory tissues. Aquat Toxicol 68:263–275
Jansson L, Falkmer S (1998) Blood flow to the pancreatic islet parenchyma of the Atlantic hagfish (Myxine glutinosa). Horm Metab Res 30:182–187
Kobayashi H, Uemura H, Takei Y, Itatsu N, Ozawa M, Ichinohe K (1983) Drinking induced by angiotensin II in fishes. Gen Comp Endocrinol 49:295–306
Liew HJ, Sinha AK, Nawata CM, Blust R, Wood CM, De Boeck G (2013) Differential responses in ammonia excretion, sodium fluxes and gill permeability explain different sensitivities to acute high environmental ammonia in three freshwater teleosts. Aquat Toxicol 126:63–76
Lomholt JP, Franko-Dossar F (1998) The sinus system of hagfish—lymphatic or secondary circulatory system? In: Jørgensen JM, Lomholt JP, Weber RE, Malte H (eds) The Biology of Hagfishes. Chapman & Hall, London, pp 259–272
Martini F (1998) The ecology of the hagfishes. In: Jørgensen JM, Lomholt JP, Weber RE, Malte H (eds) The biology of hagfishes. Chapman & Hall, London, pp 57–77
McCarthy JE (1976) Vascular and extravascular fluid volumes in the Pacific Hagfish, Eptatretus stoutii (Lockington). Unpublished M. Sc. thesis. Oregon State University, Corvallis, OR
McFarland WN, Munz FW (1958) A re-examination of the osmotic properties of the Pacific hagfish, Polistotrema stouti. Biol Bull 114:348–356
McFarland WN, Munz FW (1965) Regulation of body weight and serum composition by hagfish in various media. Comp Biochem Physiol 14:383–398
McInerney JE (1974) Renal sodium reabsorption in the hagfish, Eptatretus stouti. Comp Biochem Physiol A 49:273–280
Morris R (1965) Studies on salt and water balance in Myxine glutinosa (L.). J Exp Biol 42:359–371
Motais R, Isaia J, Rankin JC, Maetz J (1969) Adaptive changes of the water permeability of the teleostean gill epithelium in relation to external salinity. J Exp Biol 51:529–546
Nikinmaa M, Tufts BL, Boutilier RG (1993) Volume and pH regulation in agnathan erythrocytes- comparisons between the hagfish, Myxine glutinosa, and the lampreys, Petromyzon marinus and Lampetra fluviatilis. J Comp Physiol B 163:608–613
Nobata S, Ando M, Takei Y (2013) Hormonal control of drinking behavior in teleost fishes; insights from studies using eels. Gen Comp Endocrinol 192:214–221
Rankin JC (2002) Drinking in hagfishes and lampreys. In: Hazon N, Flik G (eds) Osmoregulation and drinking in vertebrates. BIOS Scientific Publishers, Oxford, pp 1–17
Riegel JA (1998) Analysis of fluid dynamics in perfused glomeruli of the hagfish Eptatretus stouti (Lockington). J Exp Biol 201:3097–3104
Robertson LM, Wood CM (2014) Measuring gill paracellular permeability with polyethylene glycol-4000 in freely swimming trout: proof of principle. J Exp Biol 217:1425–1429
Robertson LM, Kochhann D, Bianchini A, Matey V, Almeida-Val VF, Val AL, Wood CM (2015) Gill paracellular permeability and the osmorespiratory compromise during exercise in the hypoxia-tolerant Amazonian oscar (Astronotus ocellatus). J Comp Physiol B 185:741–754
Rudy PP, Wagner RC (1970) Water permeability in the Pacific hagfish Polistostrema stouti and the staghorn sculpin Leptocottus armatus. Comp Biochem Physiol 34:399–403
Sardella BA, Baker DW, Brauner CJ (2009) The effects of variable water salinity and ionic composition on the plasma status of the Pacific hagfish (Eptatretus stoutii). J Comp Physiol B 179:721–728
Schultz A, Guffey SC, Clifford AM, Goss GG (2014) Phosphate absorption across multiple epithelia in the Pacific hagfish (Eptatretus stoutii). Am J Physiol- Reg Integ Comp Physiol 307:R643–R652
Shephard KL (1994) Functions for fish mucus. Rev Fish Biol Fish 4:401–429.
Smith CJ, Shaw BJ, Handy RD (2007) Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol 82:94–109
Tait LW, Simpson CWC, Takei Y, Forster ME (2009) Hagfish natriuretic peptide changes urine flow rates and vascular tensions in a hagfish. Comp Biochem Physiol C 150:45–49
Takei Y (2015) From aquatic to terrestrial life: evolution of the mechanisms for water acquisition. Zool Sci 32:1–7
Toop T, Evans DH (1993) Whole animal volume regulation in the Atlantic hagfish, Myxine glutinosa, exposed to 85% and 115% sea water. Bull Mt Des Isl Biol Lab 32:98–99
Weinrauch AM, Edwards SL, Goss GG (2016) Anatomy of the Pacific hagfish (Eptatretus stoutii). In: Edwards SL, Goss GG (eds) Hagfish Biology. CRC Press, Boca Raton, FL, pp 1–40
Acknowledgements
The authors are grateful to Dr. Eric Clelland at BMSC for facilitating this research. Financial support was provided by Natural Sciences and Engineering Research Council of Canada Discovery grants to GGG and CMW, and by an award from the International Development Research Centre (IDRC, Canada) to CMW and Dr. Adalto Bianchini. CNG is supported by a Campus Alberta Innovates Program Research Chair.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Glover, C.N., Wood, C.M. & Goss, G.G. Drinking and water permeability in the Pacific hagfish, Eptatretus stoutii . J Comp Physiol B 187, 1127–1135 (2017). https://doi.org/10.1007/s00360-017-1097-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1097-2