Abstract
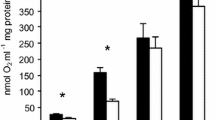
Hibernation elicits a major reduction in whole-animal O2 consumption that corresponds with active suppression of liver mitochondrial electron transport capacity at, or downstream of, succinate dehydrogenase (SDH). During arousal from the torpor phase of hibernation this suppression is reversed and metabolic rates rise dramatically. In this study, we used the 13-lined ground squirrel (Ictidomys tridecemlineatus) to assess isolated liver mitochondrial respiration during the torpor phase of hibernation and various stages of arousal to elucidate a potential role of SDH in metabolic suppression. State 3 and state 4 respiration rates were seven- and threefold lower in torpor compared with the summer-active and interbout euthermic states. Respiration rates increased during arousal so that when body temperature reached 30°C in late arousal, state 3 and state 4 respiration were 3.3- and 1.8-fold greater than during torpor, respectively. SDH activity was 72% higher in interbout euthermia than in torpor. Pre-incubating with isocitrate [to alleviate oxaloacetate (OAA) inhibition] increased state 3 respiration rate during torpor by 91%, but this rate was still fourfold lower than that measured in interbout euthermia. Isocitrate pre-incubation also eliminated differences in SDH activity among hibernation bout stages. OAA concentration correlated negatively with both respiration rates and SDH activity. These data suggest that OAA reversibly inhibits SDH in torpor, but cannot fully account for the drastic metabolic suppression observed during this hibernation phase.







Similar content being viewed by others
Abbreviations
- CS:
-
Citrate synthase
- CoQ:
-
Coenzyme Q
- ETC:
-
Electron transport chain
- IMS:
-
Intermembrane space
- OAA:
-
Oxaloacetate
- SDH:
-
Succinate dehydrogenase
- Tb :
-
Core body temperature
References
Ackrell BAC, Kearney EB, Mayr M (1974) Role of oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem 249:2021–2027
Azzam NA, Hallenbeck JA, Kachar B (2000) Membrane changes during hibernation. Nature 407:317–318
Barger JL, Brand MD, Barnes MB, Boyer BB (2003) Tissue-specific depression of mitochondrial proton leak and substrate oxidation in hibernating arctic ground squirrels. Am J Physiol 284:R1306–R1313
Brustovetsky NN, Mayevsky EI, Grishina EV, Gogvadze VG, Amerkhanov ZG (1989) Regulation of the rate of respiration and oxidative phosphorylation in liver mitochondria from hibernating ground squirrels, Citellus undulatus. Comp Biochem Physiol 94B:537–541
Chance B, Hagihara B (1962) Activation and inhibition of succinate oxidation following adenosine diphosphate supplements to pigeon heart mitochondria. J Biol Chem 237:3540–3545
Corwin LM, Schwarz K (1959) An effect of vitamin E on the regulation of succinate oxidation in rat liver mitochondria. J Biol Chem 234:191–197
Cremel G, Rebel G, Canguilhem B, Rendon A, Waksman A (1979) Seasonal variation of the composition of membrane lipids in liver mitochondria of the hibernator Cricetus cricetus: relation to intramitochondrial intermembrane protein movement. Comp Biochem Physiol 63A:159–167
Fedotcheva NI, Sharyshev AA, Mironova GD, Kondrashova MN (1985) Inhibition of succinate oxidation and K+ transport in mitochondria during hibernation. Comp Biochem Physiol 82B:191–195
Gehnrich SC, Aprille JR (1988) Hepatic gluconeogenesis and mitochondrial function during hibernation. Comp Biochem Physiol 91B:11–16
Geiser F, Ruf TP (1995) Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Geiser F, McAllen BM, Kenagy GJ (1994) The degree of dietary fatty acid unsaturation affects torpor patterns and lipid composition of a hibernator. J Comp Physiol B 164:299–305
Gerson AR, Brown JCL, Thomas R, Bernards MA, Staples JF (2008) Effects of dietary polyunsaturated fatty acids on mitochondrial metabolism in mammalian hibernation. J Exp Biol 211:2689–2699
Green JD, Narahara HT (1980) Assay of succinate dehydrogenase activity by the tetrazolium method: evaluation of an improved technique in skeletal muscle fractions. J Histochem Cytochem 28:408–412
Gutman M (1978) Modulation of mitochondrial succinate dehydrogenase activity, mechanism and function. Mol Cell Biochem 20:41–60
Gutman M, Silman N (1975) The steady state activity of succinate dehydrogenase in the presence of opposing effectors. II. Reductive activation of succinate dehydrogenase in presence of oxaloacetate. Mol Cell Biochem 7:177–185
Hazel JR (1972) The effect of temperature acclimation upon succinate dehydrogenase activity from the epaxial muscle of the common goldfish (Carassius auratus l.)–II. Lipid reactivation of the soluble enzyme. Comp Biochem Physiol 43B:863–882
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227
Helgen KM, Cole FR, Helgen LE, Wilson DE (2009) Generic Revision in the Holarctic Ground Squirrel Genus Spermophilus. J Mammal 90:270–305
Lyman CP, Willis JS, Malan A, Wang LCH (1982) Hibernation and torpor in mammals and birds. Academic Press, New York
Malatesta M, Battistelli S, Rocchi MBL, Zancanaro C, Fakan S, Gazzanelli G (2001) Fine structural modifications of liver, pancreas and brown adipose tissue mitochondria from hibernating, arousing and euthermic dormice. Cell Biol Int 25:131–138
Martin AW, Furhman FA (1955) The relationship between summated tissue respiration and metabolic rate in the mouse and dog. Physiol Zool 27:18–28
Martin SL, Maniero GD, Carey C, Hand SC (1999) Reversible Depression of Oxygen Consumption in Isolated Liver Mitochondria during Hibernation. Physiol Biochem Zool 72:255–264
McMurchie EJ, Raison JK (1979) Membrane lipid fluidity and its effect on the activation energy of membrane-associated enzymes. Biochim Biphys Acta 554:364–374
Muleme HM, Walpole AC, Staples JF (2006) Mitochondrial metabolism in hibernation: metabolic supression, temperature effects and substrate preferences. Physiol Biochem Zool 79:474–483
Munujos P, Coll-Cantí J, González-Sastre F, Gella FJ (1993) Assay of succinate dehydrogenase activity by a colorimetric-continuous method using iodonitrotetrazolium chloride as electron acceptor. Anal Biochem 212:506–509
Page MM, Peters CW, Staples JF, Stuart JA (2009) Intracellular antioxidant enzymes are not globally upregulated during hibernation in the major oxidative tissues of the 13-lined ground squirrel Spermophilus tridecemlineatus. Comp Biochem Physiol 152:115–122
Paradies G, Ruggiero FM, Dinol P, Petrosillo G, Quagliariello E (1993) Decreased cytochrome oxidase activity and changes in phospholipids in heart mitochondria from hyperthyroid rats. Arch Biochem Biophys 307:91–95
Parrilla R, Ayuso-Parrilla MS (1976) Cellular metabolite distribution and the control of gluconeogenesis in the perfused isolated rat liver. Pflügers Arch 362:49–54
Pehowich DJ (1994) Modification of skeletal muscle sarcoplasmic reticulum fatty acyl composition during arousal from hibernation. Comp Biochem Physiol 109B:571–578
Pehowich DJ, Wang LCH (1984) Seasonal changes in mitochondrial succinate dehydrogenase activity in a hibernator, Spermophilus richardsonii. J Comp Physiol 154B:495–501
Petit PX, O’Connor JE, Grunwald D, Brown SC (1990) Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem 194:389–397
Platner WS, Patnayak BC, Musacchia XJ (1972) Seasonal changes in the fatty acid spectrum in the hibernating and non-hibernating ground squirrel Citellus tridecemlineatus. Comp Biochem Physiol 42A:927–938
Rej R (1985) Oxaloacetate. In: Bergmeyer J, Grassl M (eds) Methods of Enzymatic Analysis Vol VII. Verlag Chemie, Weinheim, pp 55–66
Roberts JC, Chaffee RRJ (1972) Suppression of mitochondrial respiration in hibernation and its reversal in arousal. In: Smith RE, Shields JL, Hannon JP, Horwitz BA (eds) Proceedings of the international symposia on environmental physiology: bioenergetics and temperature regulation. FASEB, Bethesda, pp 101–107
Rolfe DFS, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758
Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL (2007) Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31:15–24
Siess EA, Brocks DG, Lattke HK, Wieland OH (1977) Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J 166:225–235
Staples JF, Buck LT (2009) Matching cellular metabolic supply and demand in energy-stressed animals. Comp Biochem Physiol A 153:95–105
Swan H, Schatte C (1976) Phospholipid fatty acids of cerebral cortex, heart and liver in the hibernating and active ground squirrel, Cittelus tridecemlineatus: a preliminary report. Am Nat 111:802–806
Takaki M, Nakahara H, Kawatani Y, Utsumi K, Suga H (1997) No suppression of respiratory function of mitochondria isolated from the hearts of anesthetized rats with high-dose pentobarbital sodium. Jpn J Physiol 47:87–92
Tyler DB (1960) Effect of citric-acid cycle intermediates on oxaloacetate utilization and succinate oxidation. Biochem J 76:293–297
Vaughan DK, Gruber AR, Michalski ML, Seidling J, Schlink S (2006) Capture, care, and captive breeding of 13-lined ground squirrels, Spermophilus tridecemlineatus. Lab Anim 35:33–40
Acknowledgments
The authors wish to express their gratitude for financial support (Discovery Grants) from the Natural Sciences and Engineering Research Council (Canada). Alvin Iverson and his staff at the University of Manitoba Carman and Region Facility were very helpful in trapping animals. Jason C.L. Brown was very helpful with his assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Armstrong, C., Staples, J.F. The role of succinate dehydrogenase and oxaloacetate in metabolic suppression during hibernation and arousal. J Comp Physiol B 180, 775–783 (2010). https://doi.org/10.1007/s00360-010-0444-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0444-3