Abstract
Stress assessment favours methods, which do not interfere with an animal’s endocrine status. To develop such non-invasive methods, detailed knowledge about the excretion of hormone metabolites in the faeces and urine is necessary. Our study was therefore designed to generate basic information about catecholamine excretion in rats, mice and chickens. After administration of 3H-epinephrine or 3H-norepinephrine to male and female rats, mice and chickens, all voided excreta were collected for 4 weeks, 3 weeks or for 10 days, respectively. Peak concentrations of radioactivity appeared in one of the first urinary samples of mice and rats and in the first droppings in chickens 0.2–7.2 h after injection. In rats, between 77.3 and 95.6% of the recovered catecholamine metabolites were found in the urine, while in mice, a mean of 76.3% were excreted in the urine. Peak concentrations in the faeces were found 7.4 h post injection in mice, and after about 16.4 h in rats (means). Our study provides valuable data about the route and the profile of catecholamine excretion in three frequently used species of laboratory animals. This represents the first step in the development of a reliable, non-invasive quantification of epinephrine and norepinephrine to monitor sympatho-adrenomedullary activity, although promising results for the development of a non-invasive method were found only for the chicken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In response to stressors, an animal’s central nervous system triggers physiological responses that ultimately result in an activation of the hypothalamic-pituitary-adrenocortical axis and/or the sympatho-adrenomedullary system, and that are presumed to have adaptive values during periods of stress as they help in regaining homeostasis (Moberg 2000; Romero 2004).
Studies assessing endocrine stress responses comparing, for example, different housing conditions, effects of routine management procedures or transportation, require non-invasive methods for hormone level measurement as traditional approaches of data collection (e.g., blood sampling) cause an additional stress response due to direct human interference (Freeman and Manning 1979; Harvey et al. 1980; Möstl and Palme 2002). Recent studies have elucidated the metabolism and excretion of glucocorticoids in different species of mammals and birds (reviewed by Palme et al. 2005). This knowledge has led to the development of novel non-invasive methods to monitor adrenocortical activity by measuring faecal metabolites of glucocorticoids (Möstl and Palme 2002; Palme 2005; Touma and Palme 2005).
However, in contrast to glucocorticoids, little is known about catecholamine (CA) excretion. CA are secreted within seconds after disturbance. Therefore, it is almost impossible to determine plasma concentrations of CA without the interference caused by the manipulation of an animal (Le Maho et al. 1992). A non-invasive method for measuring CA and/or catecholamine metabolites (CAM) in the faeces, analogous to steroid hormones, would thus be a valuable tool as the activity of the sympatho-adrenomedullary axis could be monitored without the need to directly interfere with the animal. This would enable long-term studies including frequent sampling and would also facilitate working with species that are difficult to handle.
Until now CAM have been measured in the urine of humans (Nikolajsen and Hansen 2001), dogs (Durocher et al. 2007), cats (Claustre and Peyrin 1982; Kojima et al. 1995), rats (Hay et al. 1997), cattle (Strasser et al. 1993) and pigs (Hay and Mormede 1998; Hay et al. 2000, 2003). However, the collection of urine samples is not completely non-invasive and much more difficult to perform, in particular in small laboratory animals such as mice and rats. Additionally, CA are not very stable, i.e. requiring the urine to be preserved or frozen immediately after collection. Due to these constraints, assessment of sympatho-adrenomedullary system activity in animals has often been performed indirectly, by measuring for example the blood pressure or the heart rate of an animal (Von Holst 1998). But these methods provide only an imperfect solution for monitoring sympatho-adrenomedullary system activity, since they are not non-invasive, rendering the measurement of CAM in the faeces a more suitable approach.
In order to establish a reliable method to quantify hormone metabolites in the faeces, it is essential to gather information about the time course (the time-lag until the hormone metabolites appear in the faeces) and the percentage excreted via the faeces and the urine, respectively. Both parameters are known to differ largely between species and even between sexes (Palme et al. 1996, 2005). Radioinfusion experiments in ruminants and pigs have shown that 3H–CA are mainly (>97.5%) excreted via the urine (Payne 1992; El-Bahr et al. 2005), which is also the main route of elimination in humans (Moleman et al. 1992).
In the present study, we therefore aimed to gain necessary knowledge about metabolism and excretion of CA in two species that are widely used as laboratory animals, namely mice and rats. In addition, we wanted to investigate the suitability of chicken droppings for measuring CAM, since urine and faeces are excreted together as droppings in most bird species (Klasing 2005). The purpose of this study was to investigate excretion patterns in rats, mice and chickens after intravenous (rats and chickens) or intraperitoneal (mice) administration of radioactively labelled epinephrine (E) and norepinephrine (NE).
Materials and methods
Animals and general housing conditions
The experiments were performed together with (or under the same conditions as) the 3H-corticosterone radiometabolism studies in the respective species (rats: Lepschy et al. 2007; mice: Touma et al. 2003; chickens: Rettenbacher et al. 2004).
Rats
In total, 24 twelve-week-old rats [12 males and 12 females; Fischer, Crl:CD (SD), Charles River, Germany] were used (N = 6 for each hormone and sex). The animals arrived in the laboratory four days before the experiment began. Each rat was housed individually in a Type IV standard Makrolon cage (Tecniplast, Buguggiate, Varese, Italy) with sawdust as bedding material. The animal housing room was maintained under standard laboratory conditions (light–dark cycle: 12:12 h, lights on at 6 am; temperature: 21 ± 1°C; relative humidity: 50 ± 10%). Commercial rat diet and bottled tap water were available ad libitum. Permission to conduct the animal experiments was obtained from the provincial government of Lower Austria (LF1-TVG-5/075-2004).
Mice
In total, 32 adult C57BL/6J mice (Mus musculus f. domesticus, 16 males and 16 females) were used (N = 8 for each hormone and sex). The animals were housed in same-sex groups of four individuals until the age of 9 weeks under standardized laboratory conditions (see above). Commercial mouse diet (Altromin No. 1324, Altromin GmbH, Lage, Germany; content: protein 19%, fat 4%, raw fibre 6%, ash 7%, vitamins and minerals) and bottled tap water were available ad libitum. At 10 weeks of age, the groups were separated and the mice were housed individually for the following 3 weeks under the same conditions as described above to habituate them to single housing required for the experiment. The experiments were approved by the provincial government of Muenster (23.0835.1.0 G 63/2000) as well as by the Animal Welfare Officer at the University of Muenster.
Chickens
In total, 20 (ten of each sex) mature ISA brown laying hybrids obtained from a commercial breeder (R. Schropper PLC, Gloggnitz, Austria) were used. All animals were housed individually in cages to which they were habituated one week prior to the experiments. Males and females were housed in separate rooms. Food and water were supplied ad libitum throughout the experiments. The daily light period was from 6:00 to 20:00. Permission for conducting the animal experiment was obtained from the Federal Ministry of Education, Science and Culture (GZ 68.205/59-Pr/4/2002).
Administration of radiolabelled E and NE and sample collection
All animals received 3H–E (special synthesis, WS03DC, E, levo-[ring-2,5,6-3H]) or 3H–NE (NET 678, NE, levo-[ring-2,5,6-3H]), both purchased from Perkin Elmer, Life and Analytical Sciences (Boston, MA) with a specific activity of 40–80 Ci/mmol (purity > 97%).
Rats
At 9 am on day 0 of the experiment, each rat was injected intravenously with 1.1 MBq of 3H–E or 3H–NE diluted in 0.4 ml of sterile isotonic saline solution. The whole procedure of catching, fixation, intravenous injection and transferring the rat into its individual metabolism cage (steel wire, cylindrical shape, 20 cm height, 20 cm diameter) did not exceed 4 min per animal.
All excreta dropped through the bars of the wire floor of the metabolic cages and then were separated by two pipes, one covered by a mesh that only urine could flow through. This setup enabled easy collection without disturbing the animals. Sample collections performed during the dark phase were done under dimmed light conditions to avoid disrupting the animals’ natural activity cycles. During the first 4 h, the rats were kept without water or pelleted food strictly in order to avoid contamination of the urine and faeces. After this period, bottled water was supplied ad libitum. After 12 h, food was given ad libitum as well. During the first 24 h, every voided urine and faecal sample was collected separately, stored at −24°C and the time of sampling was documented. Further sampling was done at 30, 36, 48, 72 and 96 h post injection. The animals were then put back into Type IV standard Makrolon cages and transferred into the metabolism cages only once a week (for 6 h) for 4 weeks to collect faecal and urine samples.
Mice
On day 0 of the experiment, each mouse was given an intraperitoneal injection of 0.67 MBq 3H–E or 0.33 MBq 3H–NE diluted in 1 ml of sterile isotonic saline solution containing 10% (v/v) ethanol. The whole manipulation lasted a maximum time of 3 min per animal.
The mice were housed singly in stainless steel type III wire cages, described by Touma et al. (2003), to enable individual sampling and quantitative collection of all voided urine and faeces without handling the animal. To habituate the mice to the housing and sampling procedures, the animals were placed into this housing system three days before the injections, and samples were collected in 12 h intervals during this time. Since mice, like rats, are nocturnal animals, all sampling conducted during the dark phase was performed under dimmed light conditions (<5 lux).
For 5 days after the injection, all faecal and urine samples were collected quantitatively and stored at −24°C until analysis. During the first 24 h, sampling was done according to the following time schedule: 0, 2, 4, 6, 8, 10, 12, 14, 16, 20, 24 h post injection. After the first 24 h, excreta were sampled in intervals of 12 h through day 5 of the experiment. From day 6 to day 14 after hormone administration, sampling was done daily.
Chickens
Male and female birds were administered 1.85 MBq of 3H–NE, and after an interval of 4 weeks, the same animals were administered 1.85 MBq of 3H–E. The hormones were dissolved in 1 ml of 0.9% NaCl solution containing 10% (v/v) ethanol and injected into the wing vein (Vena cutanea ulnaris). Before injection, droppings of each bird were collected to determine background levels of radioactivity. During the first 24 h after injection, all samples were collected immediately after voidance, put into plastic freezer bags and stored at −24°C. On the following day, all droppings were collected at 6 h intervals. Starting on the third day, samples were collected once a day for 10 days.
Extraction and determination of radiolabelled E and NE metabolites
Rats and mice
To determine the radioactivity in the urine samples of the rats, a total of 0.1 ml of each urine sample was put in a scintillation vial (Art. No. 6008117, Packard Instruments, Meriden, CT, USA). For the mice, the spots where urine dropped onto the filter paper were cut out, the strips of each sample were put in a scintillation vial and mixed with 2 ml of 80% methanol. Afterward, 12 ml of scintillation fluid (Quicksafe A, No. 100800, Zinnser Analytic, Maidenhead, UK) were added and the radioactivity was measured in a liquid scintillation counter (Tri-Carb 2100TR, Packard Instruments, Meriden, CT, USA; for details, see Touma et al. 2003).
To extract and determine the 3H–CAM in the faeces, each faecal sample was well homogenized (with mortar and pestle), and an aliquot of 0.1 g was suspended in a mixture of 1.6 ml of 100% methanol and 0.4 ml acetic acid (0.2 mol/l) for rat samples. For mouse samples, 0.05 g of homogenized faecal matter was suspended in 0.8 ml of 100% methanol and 0.2 ml acetic acid (0.2 mol/l). After vortexing for 30 min, each sample was centrifuged (15 min at 2,500g; Touma et al. 2003). After centrifugation, a 0.1 ml aliquot of the supernatant (in duplicate) was measured in a liquid scintillation counter (see above) and the amount of radioactivity was then calculated as described previously by Touma et al. (2003).
Chickens
A total of 0.5 g of well-homogenised droppings were suspended in a mixture of 3 ml 100% methanol and 2 ml acetic acid (0.2 mol/l). After vortexing for 30 min, the mixture was centrifuged at 3,000g for 15 min. An aliquot (0.5 ml) of the supernatant (in duplicate) was measured in a liquid scintillation counter as described previously (Rettenbacher et al. 2004).
Statistical analysis
All statistical tests were applied using the software package SigmaStat (3.11). Two independent samples were compared by using the Students t test (two tailed) when normally distributed, and by using the Mann–Whitney Rank Sum-test (two tailed) when not normally distributed. Differences were considered significant if their probability of occurring by chance was less than 5%. Mean total recovery, excretion patterns and time of peak excretion in urine and faecal samples (only applicable for rats and mice) of males and females were also statistically analysed for significant differences between the sexes.
Results
Recovery of radioactivity, median excretion and excretion maxima (peak excretion after injection time) of rats, mice and chickens treated with 3H–E or 3H–NE are given in Table 1. The total recovery of radioactivity (in rats and mice calculated as the sum of recovered radioactivity in urine and faeces) is expressed as the percentage of administered radioactivity.
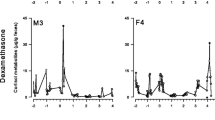
In all tested animals, the majority of the radioactivity was excreted within the first 24 h, while the remaining radioactivity was excreted slowly over the following days/weeks until background levels were reached (see Figs. 1 and 2).
Rats
After injection of E, peak radioactivity appeared in the first urine samples following administration (median: 3.0 h, range: 1.2–6.2 h in males and females, Table 1), and subsequently decreased rapidly (see Fig. 1). Nevertheless, background levels were reached only after 5 weeks. In the faeces, maximum radioactivity was found about 18 h post injection (range: 13.0–21.3 h; Table 1). The major part of the injected radioactivity was recovered in the urine (median: 88.9%, range: 77.3–92.3%; Table 1), while only small amounts (median: 11.1%, range: 7.7–22.7%) were recovered in the faeces. There were no significant differences between sexes regarding these parameters.
In the NE experiment, the mean total recovery in males was significantly higher than that in females [82.4 ± 5.1 vs. 68 ± 5.2, t test, n (males) = n (females) = 6; P < 0.001]. Peak radioactivity in urine samples appeared 1.0–7.2 h post injection and in faecal samples after 9.1–18.8 h in both sexes (see Fig. 2). Compared to recovery of E, total recovery in the urine was higher (90.9–95.6%).
Mice
Excretion patterns of E and NE in mice were in general similar to the findings for rats, but a larger inter-individual variation was observed, in particular within the females (see Table 1).
In the E experiment, the mean total recovery of radioactive CAM was relatively high for both sexes, ranging between 73.8 and 99.3%. Excretion of peak radioactivity in the urine usually occurred in the first urinary samples, i.e., those collected two hours after the injection (males and females: median: 2 h, range: 2–4 h). Peak radioactivity in faeces was recovered later in both sexes, but the timing differed significantly between males and females (males: median: 8 h, range: 8–10 h, females: median: 6 h, range: 2–12 h; Mann–Whitney Rank Sum Test, n (males) = n (females) = 8; P = 0.028). The route of excretion also differed significantly between sexes [Mann–Whitney Rank Sum Test, n (males) = n (females) = 8; P = 0.028]. In males, all except one individual excreted the majority of radioactivity via the urine (median: 91.0%, range: 23.4–93.9%), whereas the percentage of radioactive CAM in the urine of females was lower and two of the eight individuals even excreted higher percentages via the faeces (median: 84.0%, range: 10.1–90.6%). Thus, the percentage of excreted E metabolites in the faeces was relatively low (with a few exceptions) in males as well as in females.
Similar results were obtained in the NE experiment (see Table 1). Again, the total recovery ranged between 70.8 and 99.6% for both sexes and the majority of radioactive NE metabolites was excreted via the urine. As in the E experiment, urinary excretion of CAM in males was more consistent than in females (males: median: 94.7%, range: 92.7–96.1%; females: median: 68.7%, range: 11.1–96.9%), although not statistically significant between the sexes. In general, peak radioactivity was recovered within 2 h in the urine (males: median: 3 h, range: 2–4 h; females: 2 h, range: 2–6 h). In the faeces, the time delay ranged from 6 to 14 h in males (median: 8 h) and from 2 to 8 h in females (median: 7 h). Thus, in contrast to the E experiment, the sexes did not differ significantly regarding the route and the delay time of NE metabolite excretion.
Chickens
For both hormones, significant differences between the sexes concerning the time of excretion maxima were not detected. Following E administration, peak excretion occurred within 1.0 h (range 0.2–1.9 h) in both males and females. In two males and three females, a second, smaller peak was observed (see Fig. 1). Individual data are given in Table 1. The median total recovery was 62.2% in both sexes (range: 45.4–104.3%).
In the NE trial, excretion maxima were reached after 0.7 h (range: 0.2–1.7 h) in both males and females. Six males and three females showed a small second peak (see Fig. 2). The median total recovery was 70.5% in both sexes (range: 41.6–95.2%). Timing of the excretion maxima differed significantly between the E and NE trial [t test, n (males) = n (females) = 10; P = 0.048).
Discussion
The goal of this study was to gather basic information about the excretion of CA in three species of commonly used laboratory animals. Therefore, 3H–E and 3H–NE were administered to mice, rats and chickens. All voided excreta were collected and radioactivity of each sample was measured.
The overall percentage of recovered radioactivity was comparable to experiments on the metabolism of steroids (Palme et al. 2005) and on the metabolism of CA in domestic livestock (El-Bahr et al. 2005). In rats, however, background levels were reached later than after administration of glucocorticoids (Lepschy et al. 2007). The formation of adducts, the binding of CA to plasma proteins, could be a possible explanation for this. Adduct formation in vitro has been reported not only for rats (Powis 1975a), but for several other species including humans (Powis 1975b; Sager et al. 1987), dogs (Teixeira et al. 1979), sheep (El-Bahr et al. 2006) and domestic fowl (Powis 1975a). The uptake and/or binding of CA in blood cells offers further explanations for delayed excretion of 3H–CA and was demonstrated in humans (Altman et al. 1988; Azoui et al. 1994, 1996a; Ratge et al. 1991; Born et al. 1967), sheep (El-Bahr et al. 2006), rabbits (Blakeley and Nicol 1978; Friedgen et al. 1993) and rats (Azoui et al. 1996b; Alexander et al. 1984; Bouvier et al. 1987; Yoneda et al. 1984). The significantly lower recovery in female rats in the NE trial may also be attributed to these mechanisms.
In the mouse and chicken experiments, sampling was performed only for 2 weeks and 10 days after the injection, respectively. Therefore, a similar phenomenon could not be investigated. However, the lower recovery rates compared to radiometabolism studies of glucocorticoid performed in the same species (Touma et al. 2003; Rettenbacher et al. 2004) indicate similar mechanisms. Future studies should address possible interactions of CA with proteins in the blood of both mice and chickens.
In mice and rats, radioactive CAM were mainly excreted via the urine, but the faecal proportion was larger than in sheep and pigs, which were reported to be only about 2.5% (El-Bahr et al. 2005).
After injection of 3H–E, the rats showed an excretion pattern which was similar to that of glucocorticoids (Lepschy et al. 2007). In the 3H–E experiment, the main share of the administered radioactivity (more than 80%) was excreted in the urine. These findings correspond with the results of Kopin et al. (1961), who also found only 15% of the administered H3–E in the faeces and the rest in the urine.
With regard to the distribution of radiolabelled CAM between urine and faeces, mice showed a noticeable difference in the proportions. This was more pronounced in females, where excretion in the urine varied considerably in both hormones. To our knowledge, such marked inter-individual differences of the CAM excretion patterns in females have not been described previously for any species. Cross contamination of urine and faeces can be ruled out, as the excretion peak of urine and faeces always appeared at the expected point of time and showed a distinct pattern of increase and decrease. Certainly, cross contamination would have disrupted these patterns of different points of excretion maxima in faeces and urine. Failure of intraperitoneal injection might account for the high variance (Gaines Das and North 2007), too. However, this explanation seems to be unlikely as the same administration technique in previous applications did not result in variability in the amount of recovered radioactivity or in the distribution of radioactivity in the faeces or the urine of mice (Touma et al. 2003). Therefore, sex-specific adduct formation of CA, including binding to specific serum proteins has not been described previously for any species in vivo, but might be taken into consideration, since similar mechanisms, i.e., binding of corticosterone to corticosterone binding globulins are well described in several species (see Westphal 1983 for a review).
In chickens, excretion patterns were similar between individuals and also between sexes. The peak concentrations of radioactivity were excreted within 2 h after administration. Although the timing of the excretion maxima of E and NE differed significantly, these results should be interpreted with caution. Since peak radioactivity appears in the first dropping after 3H–CA injection in chickens, a slightly different defecation frequency of the E and NE groups might have led to this result. In the chicken, urine and faeces are excreted together as droppings. In individuals with a two-peaked excretion curve, the first peak may be the result of renal, the second of intestinal excretion (observed and described after glucocorticoid administration, Rettenbacher et al. 2004). The fact that the second peak was not detectable in all animals, together with the findings from mammals, indicates that in chickens, similar to mammals, urinary excretion is the main route of elimination while faecal excretion is low.
For a reliable quantification of CAM, excretion patterns must be constant in both the proportion of excreted metabolites as well as the time of occurrence. With regard to our results, these requirements are better met in rats than in mice, as the latter species shows a more variable excretion pattern, especially in the females.
In conclusion, establishing a non-invasive method for measuring CA in faeces seems to be feasible only in the chicken, which was investigated as a representative bird species. Therefore, further investigations seem promising for this species. Future studies should address the nature of the excreted metabolites to some extent in order to develop suitable antibodies for their quantification. A non-invasive method for the measurement of CAM in the excreta analogous to steroid hormones would allow monitoring of the activity of the sympatho-adrenomedullary system as well. This could serve as a valuable tool for a number of research fields and would open new perspectives for biomedical and pharmacological investigations as well as for animal welfare related studies.
References
Alexander N, Yoneda S, Vlachakis ND, Maronde RF (1984) Role of conjugation and red blood cells for inactivation of circulating CA. Am J Physiol 27:R203–R207
Altman RJ, Smith CC, Betteridge J (1988) CA content of human erythrocytes. Clin Chem 34:2120–2122
Azoui R, Vignon D, Safar M, Cuche JL (1994) Plasma erythrocyte relationship of CA in human blood. J Cardiovasc Pharmacol 23:525–531
Azoui R, Cuche JL, Renaud JF, Safar M, Dagher G (1996a) A dopamine transporter in human erythrocytes: modulation by insulin. Exp Physiol 81:421–434
Azoui R, Schneider J, Dong WX, Dabire H, Safar M, Cuche JL (1996b) Red blood cells participate in the metabolic clearance of CA in the rat. Life Sci 60:357–367
Blakeley AG, Nicol CJ (1978) Accumulation of amines by rabbit erythrocytes in vitro. J Physiol 277:77–90
Born GV, Day M, Stockbridge A (1967) The uptake of amines by human erythrocytes in vitro. J Physiol 193:405–418
Bouvier M, Farley L, de Champlain J (1987) Red blood cell CA levels in normotensive and DOCA-salt hypertensive rats. Am J Physiol 253:H270–H275
Claustre J, Peyrin L (1982) Free and conjugated CA and metabolites in cat urine after hypoxia. J Appl Physiol 52:304–308
Durocher LL, Hinchcliff KW, Williamson KK, McKenzie EC, Holbrook TC, Willard M, Royer CM, Davis MS (2007) Effect of strenuous exercise on urine concentrations of homovanillic acid, cortisol, and vanillylmandelic acid in sled dogs. Am J Vet Res 68:107–111
El-Bahr SM, Kahlbacher H, Rausch WD, Palme RG (2005) Excretion of CA (adrenaline and noradrenaline) in domestic livestock. Vet Med Austria/Wien Tierarztl Mschr 92:207–213
El-Bahr SM, Kahlbacher H, Patzl M, Palme RG (2006) Clearance and binding systems of radiolabelled adrenaline and noradrenaline in sheep blood. Vet Res Commun 30:423–432
Freeman BM, Manning AC (1979) Stressor effects of handling on the immature fowl. Res Vet Sci 26:223–226
Friedgen B, Halbrugge T, Graefe KH (1993) Plasma clearances and extractions of four CA in the anesthetized rabbit: the role of amine removal by blood cells. J Cardiovasc Pharmacol 21:21–28
Gaines Das R, North D (2007) Implication of experimental technique for analysis and interpretation of data from increased variability resulting from failure of intraperitoneal injection procedures. Lab Anim UK 41:312–320
Harvey S, Merry BJ, Phillips JG (1980) Influence of stress on the secretion of corticosterone in the duck (Anas-Platyrhynchos). J Endocrinol 87:161–171
Hay M, Mormede P (1998) Urinary excretion of CA, cortisol and their metabolites in Meishan and large white sows: validation as a non-invasive and integrative assessment of adrenocortical and sympathoadrenal axis activity. Vet Res 29:119–128
Hay M, Meunier-Salaun MC, Brulaud F, Monnier M, Mormede P (1997) Assessment of hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity in pregnant sows through the measurement of glucocorticoids and CA in urine. J Anim Sci 78:420–428
Hay M, Meunier-Salaun MC, Brulaud F, Monnier M, Mormede P (2000) Assessment of hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity in pregnant sows through the measurement of glucocorticoids and CA in urine. J Anim Sci 78:420–428
Hay M, Vulin A, Genin S, Sales P, Prunier A (2003) Assessment of pain induced by castration in piglets: behavioral and physiological responses over the subsequent 5 days. Appl Anim Behav Sci 82:201–218
Klasing KC (2005) Potential impact of nutritional strategy on noninvasive measurements of hormones in birds. Ann NY Acad Sci 1046:5–16
Kojima K, Maki S, Hirata K, Higuchi S, Akazawa K, Tashiro N (1995) Relation of emotional behaviors to urine CA and cortisol. Physiol Behav 57:445–449
Kopin IJ, Axelrod J, Gordon E (1961) The metabolic fate of H3-E and C14-metanephrine in the rat. J Biol Chem 36:2109–2113
Le Maho Y, Karmann H, Briot D, Handrich Y, Robin JP, Mioskowski E, Cherel Y, Farni J (1992) Stress in birds due to routine handling and a technique to avoid it. Am J Physiol 263:R775–R781
Lepschy M, Touma C, Hruby R, Palme R (2007) Non-invasive measurement of adrenocortical activity in male and female rats. Lab Anim UK 41:372–387
Moberg GP (2000) Biological response to stress: implication for animal welfare. In: Moberg GP, Mench JA (eds) The biology of animal stress. CABI Publishing, Oxon, pp 123–146
Moleman P, Tulen JH, Blankestijn PJ, Man in ‘t Veld AJ, Boomsma F (1992) Urinary excretion of CA and their metabolites in relation to circulating CA. Six-hour infusion of E and NE in healthy volunteers. Arch Gen Psychiatry 49:568–572
Möstl E, Palme R (2002) Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74
Nikolajsen RPH, Hansen AM (2001) Analytical methods for determining urinary CA in healthy subjects. Anal Chim Acta 449:1–15
Palme R (2005) Measuring fecal steroids: guidelines for practical application. Ann NY Acad Sci 1046:75–80
Palme R, Fischer P, Schildorfer H, Ismail MN (1996) Excretion of infused 14C-steroid hormones via faeces and urine in domestic livestock. Anim Reprod Sci 43:43–63
Palme R, Rettenbacher S, Touma C, El Bahr SM, Möstl E (2005) Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion, and noninvasive measurement in faecal samples. Ann NY Acad Sci 1040:162–171
Payne E (1992) The metabolism of noradrenaline in the sheep and the effect of dry matter intake upon the production of a metabolite, urinary vanillylmandelic acid. Comp Biochem Physiol C 101:661–664
Powis G (1975a) The binding of CA to the serum proteins of the rat and the domestic fowl. Comp Biochem Physiol C 52:85–90
Powis G (1975b) The binding of CA to human serum proteins. Biochem Pharmacol 24:707–712
Ratge D, Kohse KP, Steegmuller U, Wisser H (1991) Distribution of free and conjugated CA between plasma, platelets and erythrocytes: different effects of intravenous and oral CA administrations. J Pharmacol Exp Ther 257:232–238
Rettenbacher S, Möstl E, Hackl R, Ghareeb K, Palme R (2004) Measurement of corticosterone metabolites in chicken droppings. Br Poult Sci 45:704–711
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Sager G, Bratlid H, Little C (1987) Binding of CA to alpha-1 acid glycoprotein, albumin and lipoproteins in human serum. Biochem Pharmacol 36:3607–3612
Strasser A, Kruzik P, Weiser M (1993) Measurement of CA and indoleamine metabolites in urine of cattle. Wien Tierarztl Monatsschr 80:297–301
Teixeira F, Branco D, Torrinha JF (1979) Binding of adrenaline and isoprenaline to plasma proteins of the dog. Pharmacol 18:228–234
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046:54–74
Touma C, Sachser N, Möstl E, Palme R (2003) Effect of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278
Von Holst D (1998) The concept of stress and its relevance for animal behavior. Adv Study Behav 27:1–131
Westphal U (1983) Steroid-protein interaction: from past to present. J Steroid Biochem 19:1–15
Yoneda S, Alexander N, Vlachakis ND, Maronde RF (1984) Role of conjugation and red blood cells for inactivation of circulating normetanephrine. Am J Physiol 247:R208–R211
Acknowledgments
We thank the staff of the Institute of Toxicology of the Austrian Research Center Seibersdorf; the team of the Clinic for Avian, Reptile and Fish Medicine of the University of Veterinary Medicine, Vienna; and the animal caretaker team at the central animal housing facility of the University of Muenster for their skilled technical assistance during the animal experiments. The study on rats was funded by the Austrian Federal Ministry for Education, Science and Culture (GZ 80.102/2-BrGT/2004). S. Rettenbacher was supported by a “von Fircks” doctoral scholarship. C. Touma was supported by a Ph.D.-scholarship from the “Studienstiftung des deutschen Volkes.”
All animal experiments comply with the current laws of the countries in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lepschy, M., Rettenbacher, S., Touma, C. et al. Excretion of catecholamines in rats, mice and chicken. J Comp Physiol B 178, 629–636 (2008). https://doi.org/10.1007/s00360-008-0254-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0254-z