Abstract
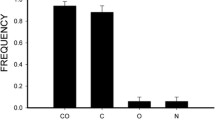
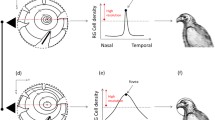
Foraging mode influences the dominant sensory modality used by a forager and likely the strategies of information gathering used in foraging and anti-predator contexts. We assessed three components of visual information gathering in a sit-and-wait avian predator, the black phoebe (Sayornis nigricans): configuration of the visual field, degree of eye movement, and scanning behavior through head-movement rates. We found that black phoebes have larger lateral visual fields than similarly sized ground-foraging passerines, as well as relatively narrower binocular and blind areas. Black phoebes moved their eyes, but eye movement amplitude was relatively smaller than in other passerines. Black phoebes may compensate for eye movement constraints with head movements. The rate of head movements increased before attacking prey in comparison to non-foraging contexts and before movements between perches. These findings suggest that black phoebes use their lateral visual fields, likely subtended by areas of high acuity in the retina, to track prey items in a three-dimensional space through active head movements. These head movements may increase depth perception, motion detection and tracking. Studying information gathering through head movement changes, rather than body posture changes (head-up, head-down) as generally presented in the literature, may allow us to better understand the mechanisms of information gathering from a comparative perspective.




Similar content being viewed by others
References
Bednekoff PA, Lima SL (2002) Why are scanning patterns so variable? An overlooked question in the study of anti-predator vigilance. J Avian Biol 33:143–149
Blackwell BF, Fernández-Juricic F, Seamans TW, Dolan T (2009) Avian visual system configuration and behavioural response to object approach. Am Behav 77:673–684
Blumstein DT, Evans CS, Daniel JC (2000) JWatcher. Available at: http://www.jwatcher.ucla.edu/
Boire D, Dufour JS, Théoret H, Ptito M (2001) Quantitative analysis of the retinal ganglion cell layer in the ostrich, Struthio camelus. Brain Behav Evol 58:343–355
Cabe PR (1993) European starling (Sturnus vulgaris). In: Pool A (ed) The birds of North America (online). Cornell Laboratory of Ornithology, Ithaca, New York. Available at The birds of North America Online database, http://bna.birds.cornell.edu/bna/species/048
Casperson LW (1999) Head movements and vision in underwater-feeding birds of steam, lake and seashore. Bird Behav 13:31–46
Coimbra JP, Marceliano MLV, Andrade-da-Costa BLV, Yamada ES (2006) The retina of tyrant flycatchers: topographic organization of neuronal density and size in the ganglion cell layer of the great kiskadee Pitangus sulphuratus and the rusty margined flycatcher Myiozetetes cayanensis (Aves: Tyrannidae). Brain Behav Evol 68:15–25
Davies MNO, Green PR (1994) Multiple sources of depth information: an ecological approach. In: Davies MNO, Green PR (eds) Perception and motor control in birds: an ecological approach. Springer, New York, pp 339–356
Dawkins MS (2002) What are birds looking at? Head movements and eye use in chickens. Am Behav 63:991–998
Dawkins MS, Woodington A (2000) Pattern recognition and active vision in chickens. Nature 403:652–655
Elgar MA (1989) Predator vigilance and group size in mammals and birds: a critical review of empirical evidence. Biol Rev 64:13–33
Fernández-Juricic E, Erichsen JT, Kacelnik A (2004) Visual perception and social foraging in birds. Trends Ecol and Evol 19:25–31. doi:101016/jtree200310003
Fernández-Juricic E, Gall MD, Dolan T, Tisdale V, Martin GR (2008) The visual fields of two ground-foraging birds, house finches and house sparrows, allow for simultaneous foraging and anti-predator vigilance. Ibis 150:779–787. doi:101111/j1474-919X200800860x
Gall MD, Fernández-Juricic E (2009) Physical and visual access to prey modifies patch selection and food search effort in a sit-and-wait predator, the black phoebe. Condor 111:150–158
Getty T, Pulliam RG (1993) Search and prey detection by foraging sparrows. Ecology 74:734–742
Gibson JJ (1986) The ecological approach to visual perception. Lawrence Erlbaum Associates, New Jersey
Guillemain M, Martin GR, Fritz H (2002) Feeding methods, visual fields and vigilance in dabbling ducks (Anatidae). Funct Ecol 16:522–529
Hill GE (1993) House Finch (Carpodacus mexicanus). In: Poole A, Gill F (eds) The birds of North America, no 46. The Academy of Natural Sciences, Philadelphia. The American Ornithologists’ Union, Washington
Huey RB, Pianka ER (1981) Ecological consequences of foraging mode. Ecology 62:991–999
Hughes A (1977) The topography of vision in mammals of contrasting life style: comparative optics and retinal organization. In: Crescitelli F (ed) The visual system of vertebrates; handbook of sensory physiology, vol VII/5. Springer, New York, pp 613–756
Jones KA, Krebs JR, Whittingham MJ (2007) Vigilance in the third dimension: head movement not scan duration varies in response to different predator models. Am Behav 74:1181–1187. doi:101016/janbehav200609029
Kral K (2003) Behavioural–analytical studies in the role of head movements and depth perception in insects, birds and mammals. Behav Process 64:1–12
Land MF (1999) The role of head movements in the search and capture strategy of a tern (Aves, Laridae). J Comp Physiol A 184:265–272
Lima SL, Bednekoff BA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lowther PE, Cink CL (2006) House Sparrow (Passer domesticus). In: Poole A (ed) The birds of North America online. Cornell Laboratory of Ornithology, Ithaca, New York. Available at The birds of North American online database, http://bna.birds.cornell.edu/BNA/account/House_Sparrow/
Maldonado PE, Maturana H, Varela FJ (1988) Frontal and lateral visual systems in birds: frontal and lateral gaze. Brain Behav Evol 32:57–62
Martin GR (1984) The visual fields of the tawny owl, Strix aluco L. Vis Res 24:1739–1751
Martin GR (1986a) The eye of a passeriform bird, the European starling (Sturnus vulgaris): eye movement amplitude, visual fields and schematic optics. J Comp Physiol A 199:545–557
Martin GR (1986b) Total panoramic vision in the mallard duck, Anas platyrhynchos. Vis Res 26:1303–1306
Martin GR (1993) Producing the image. In: Zeigler HP, Bischof H (eds) Vision brain and behavior in birds. MIT Press, Cambridge, pp 5–24
Martin GR (1999a) Optical structure and visual fields in birds: their relationship with foraging behaviour and ecology. In: Archer SN, Djamgoz MBA, Loew ER, Partridge JC, Vallerga S (eds) Adaptive mechanisms in the ecology of vision. Kluwer, The Netherlands, pp 485–508
Martin GR (1999b) Eye structure and foraging in king penguins Aptenodytes patagonicus. Ibis 141:444–450
Martin GR (2007) Visual fields and their functions in birds. J Ornithol 148:S547–S562. doi:101007/s10336-007-0213-6
Martin GR, Coetzee HI (2004) Visual fields in hornbills: precision-grasping and sunshades. Ibis 146:18–26
Martin GR, Katzir G (1994) Visual fields in the stone curlew Burhinus oedicnemus. Ibis 136:448–453
Martin GR, Katzir G (1999) Visual field in Short-toed eagles Circaetus gallicus and the function of binocularity in birds. Brain Behav Evol 53:55–66
Martin GR, Piersma T (2009) Vision and touch in relation to foraging and predator detection: insightful contrasts between a plover and a sandpiper. Proc Biol Sci 276:437–445
Martin GR, Jarrett N, Tovey P, White CR (2005) Visual fields in flamingos: chick-feeding versus filter-feeding. Naturwissenschaften 92:351–354
Martin GR, Jarrett N, Williams M (2007) Visual fields in blue ducks and pink-eared ducks: visual and tactile foraging. Ibis 149:112–120
Martin GR, White CR, Butler PJ (2008) Vision and the foraging technique of great cormorants Phalacrocorax carbo: pursuit or close-quarter foraging? Ibis 150:485–494
McFadden SA (1993) Construction the three-dimensional image. In: Zeigler HP, Bischof H (eds) Vision brain and behavior in birds. MIT Press, Cambridge, pp 47–61
Meyer DBC (1977) The avian eye and its adaptations. In: Crescitelli F (ed) The visual system of vertebrates; handbook of sensory physiology, vol VII/5. Springer, New York, pp 549–612
Nalbach HO, Thier P, Varjú D (1993) Binocular interaction in the optokinetic system of the crab Carcinus maenas (L): optokinetic gain modified by bilateral image flow. Vis Neurosci 10:873–885
Ohlendorf HM (1976) Comparative breeding ecology of phoebes in Trans-Pecos Texas. Wilson Bull 88:255–271
Sillman AJ (1973) Avian vision. In: King DS, King JR (eds) Avian biology. Academy Press, New York, pp 349–387
Vincent SE, Shine R, Brown GP (2005) Does foraging mode influence sensory modalities for prey detection in male and female filesnakes, Acrochordus arafurae? Am Behav 70:715–721
Wallman J, Letelier JC (1993) Eye movements, head movements, and gaze stabilization in birds. In: Zeigler HP, Bischof H (eds) Vision brain and behavior in birds. MIT Press, Cambridge, pp 245–263
Williams DR, Coletta NJ (1987) Cone spacing and the visual resolution limit. J Opt Soc Am 4:1514–1523
Wolf BO (1997) Black Phoebe (Sayornis nigricans). In: Poole A, Gill F (eds) The birds of North America, no 268. The Academy of Natural Sciences, Philadelphia
Acknowledgments
We would like to thank M. Wineberger, K. Malaban, and S. Thomas for their assistance with data collection. We also thank J. Lucas, M. Nolen, K. Henry, L. Brierley and the Purdue journal club for their comments on this manuscript. This work was funded by a Sigma Xi Grant-in-aid-of-Research and by National Science Foundation DBI-0641550. All work was approved by the California State University Long Beach IACUC (Protocol #220).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gall, M.D., Fernández-Juricic, E. Visual fields, eye movements, and scanning behavior of a sit-and-wait predator, the black phoebe (Sayornis nigricans). J Comp Physiol A 196, 15–22 (2010). https://doi.org/10.1007/s00359-009-0488-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-009-0488-6