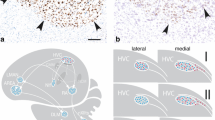
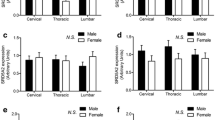
Abstract
In zebra finches, the vocal organ (syrinx) is larger in males than in females. Specific details about the mechanisms responsible for this dimorphism are not known, but may involve sex differences in steroid hormone action early in post-hatching development. The distribution of androgen receptor (AR), aromatase (AROM), estrogen receptor α (ERα), and estrogen receptor β (ERβ) mRNAs was examined at post-hatching days 3, 10 and 17. A low level of AR was equivalently expressed in the syrinx muscles of both sexes at all three ages. We detected no specific expression of AROM or ERα mRNAs. In contrast, ERβ mRNA was detected in chondrocytes of the forming bone. The density of this expression increased with age as the chondrocytes hypertrophied, but did not differ between the sexes. Taken together, these data suggest that estrogens may act on cartilage/bone, and androgens may act on muscle fibers in early post-hatching development to influence syrinx morphology. However, the lack of a sex difference in steroid receptor mRNA expression in the syrinx suggests that, similar to the forebrain regions that control song, the interaction of androgens and estrogens with their receptors is not sufficient to induce full sexual differentiation of this organ.




Similar content being viewed by others
References
Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA (1990) Sex steroid levels in developing and adult male and female zebra finches (Peophila guttata). Gen Comp Endocrinol 78:93–109
Arnold AP (1992) Developmental plasticity in neural circuits controlling birdsong: sexual differentiation and the neural basis of learning. J Neurobiol 23:1506–1528
Arnold AP (1997) Sexual differentiation of the zebra finch song system: positive evidence, negative evidence, null hypothesis, and a paradigm shift. J Neurobiol 33:572–584
Aubin JE, Liu F, Malaval L, Gupta AK (1995) Osteoblast and chondroblast differentiation. Bone 17:77S–83S
Balthazart J, Adkins-Regan E (2002) Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (eds) Hormones, brain and behavior. Academic Press, San Diego, pp 223–301
Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF (1999) Androgen receptor, estrogen receptor α, and estrogen receptor β show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology 140:4633–4643
Blanchard O, Tsagris L, Rappaport R, Duval-Beaupere G, Corvol M (1991) Age-dependent responsiveness of rabbit and human cartilage cells to sex steroids in vitro. J Steroid Biochem Mol Biol 40:711–716
Bord S, Horner A, Beavan S, Compston J (2001) Estrogen receptors α and β are differentially expressed in developing human bone. J Clin Endocrinol Metab 86:2309–2314
Corvol M-T, Carrascosa A, Tsagris L, Blanchard O, Rappaport R (1987) Evidence for a direct in vitro action of sex steroids on rabbit cartilage cells during skeletal growth: influence of age and sex. Endocrinology 120:1422–1429
van der Eerden BCJ, Gevers EF, Löwik CWGM, Karperien M, Wit JM (2002) Expression of estrogen receptor α and β in the epiphyseal plate of the rat. Bone 30:478–485
van der Eerden BCJ, Karperien M, Wit JM (2003) Systemic and local regulation of the growth plate. Endocr Rev 24:782–801
Egerbacher M, Helmreich M, Rossmanith W, Haeusler G (2002) Estrogen receptor-alpha and estrogen receptor-beta are present in the human growth plate in childhood and adolescence in identical distribution. Horm Res 58:99–103
Eroschenko VP (1996) Cartilage. In: Balado D, Vaughn VR (eds) Di Fiore’s atlas of histology with functional correlations. Williams and Wilkins, Philadelphia, pp 39–43
Godsave SF, Lohman R, Vloet RPM, Gahr M (2002) Androgen receptors in the embryonic zebra finch hindbrain suggest a function for maternal androgens in perihatching survival. J Comp Neurol 453:57–70
Goller F, Larsen ON (1997) A new mechanism of sound generation in songbirds. Proc Natl Acad Sci USA 94:14787–14791
Goller F, Larsen ON (2002) New perspectives on mechanisms of sound generation in songbirds. J Comp Physiol A 188:841–850
Grisham W, Arnold AP (1995) A direct comparison of the masculinizing effects of testosterone, androstenedione, estrogen, and progesterone on the development of the zebra finch song system. J Neurobiol 26:163–170
Gurney ME (1981) Hormonal control of cell form and number in the zebra finch song system. J Neurosci 1:658–673
Gurney ME (1982) Behavioral correlates of sexual differentiation in the zebra finch song system. Brain Res 231:153–172
Gurney ME, Konishi M (1980) Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science 208:1380–1383
Holloway CC, Clayton DF (2001) Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci 4:170–175
Horton WE Jr, Feng L, Adams C (1998) Chondrocyte apoptosis in development, aging and disease. Matrix Biol 17:107–115
Hutchison JB, Wingfield JC, Hutchison RE (1984) Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol 103:363–369
Jacobs EC, Grisham W, Arnold AP (1995) Lack of a synergistic effect between estradiol and dihydrotestosterone in the masculinization of the zebra finch song system. J Neurobiol 27:513–519
Jacobs EC, Arnold AP, Campagnoni AT (1996) Zebra finch estrogen receptor cDNA: cloning and mRNA expression. J Steroid Biochem Mol Biol 59:135–145
Karsenty G (1999) The genetic transformation of bone biology. Genes Dev 13:3037–3051
King AS (1989) Functional anatomy of the syrinx. In: King AS, McLelland J (eds) Form and function in birds, vol 4. Academic Press, New York, pp 105–192
Larsen ON, Goller F (2002) Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol 205:25–35
Leach RM, Rosselot GE (1992) The use of avian epiphyseal chondrocytes for in vitro studies of skeletal metabolism. J Nutr 122:802–805
Lieberburg I, Nottebohm F (1979) High-affinity androgen binding proteins in syringeal tissues of songbirds. Gen Comp Endocrinol 37:286–293
Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C (2001) Estrogen receptor specificity in the regulation of the skeleton in female male. J Enodocrinol 171:229–236
Lotz M, Hashimoto S, Kuhn K (1999) Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage 7:389–391
Luine V, Nottebohm F, Harding C, McEwen BS (1980) Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res 192:89–107
Luine VN, Harding CF, Bleisch WV (1983) Specificity of gonadal hormone modulation of cholinergic enzymes in the avian syrinx. Brain Res 279:339–342
Nasatzky E, Schwartz Z, Boyan BD, Soskolne WA, Ornoy A (1993) Sex-dependent effects of 17-beta-estradiol on chondrocyte differentiation in culture. J Cell Physiol 154:359–367
Newman B, Wallis GA (2003) Skeletal dysplasias caused by a disruption of skeletal patterning and endochondral ossification. Clin Genet 63:241–251
Nilsson LO, Boman A, Sävendahl L, Grigelioniene G, Ohlsson C, Ritzén EM, Wroblewski J (1999) Demonstration of estrogen receptor-β immunoreactivity in human growth plate cartilage. J Clin Endocrinol Metab 84:370–373
Nilsson LO, Abad V, Chrysis D, Ritzén EM, Sävendahl L, Baron J (2002) Estrogen receptor-α and -β are expressed throughout postnatal development in the rat and rabbit growth plate. J Endocrinol 173:407–414
Nottebohm F, Arnold AP (1976) Sexual dimorphisms in vocal control areas of the songbird brain. Science 194:211–213
Noumura T, Matsumoto E, Takahashi M (1985) On the development of sexual dimorphism in the duck syrinx and estradiol binding. In: Lofts B, Holmes WN (eds) Current trends in comparative endocrinology. Hong Kong University Press, Hong Kong, pp 601–602
Olsen BR, Reginato AM, Wang W (2000) Bone development. Annu Rev Cell Dev Biol 16:191–220
Ornoy A, Giron S, Aner R, Goldstein M, Boyan BD, Schwartz Z (1994) Gender dependent effects of testosterone and 17 beta-estradiol on bone growth and modeling in young mice. Bone Miner 24:43–58
Perlman WR, Arnold AP (2003) Expression of estrogen receptor and aromatase mRNAs in embryonic and posthatch zebra finch brain. J Neurobiol 55:204–219
Perlman WR, Ramachandran B, Arnold AP (2003) Expression of androgen receptor mRNA in the late embryonic and early posthatch zebra finch brain. J Comp Neurol 455:513–530
Rosen G, O’Bryant E, Matthews J, Zacharewski T, Wade J (2002) Distribution of androgen receptor mRNA expression and immunoreactivity in the brain of the green anole lizard. J Neuroendocrinol 14:19–28
Schlinger BA, Arnold AP (1992) Plasma sex steroids and tissue aromatization in hatchling zebra finches: implications for the sexual differentiation of singing behavior. Endocrinology 130:289–299
Schwartz Z, Meincke J, Nasatzky E, Dean DD, Boyan BD (1995) Estrogen regulation of endochondral bone formation. In: Ornoy A (ed) Animal models of human related calcium metabolic disorders. CRC Press, Boca Raton, pp 149–164
Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT (1994) Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol Brain Res 24:227–237
Simpson HB, Vicario DS (1991) Early estrogen treatment of female zebra finches masculinizes the brain pathway for learned vocalizations. J Neurobiol 22:777–793
Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Regon M, Gaillard-Kelly M, Baron R (2002) Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not males. Bone 30:18–25
Sokal RR, Rohlf FJ (1981) Biometry. WH Freeman, New York
Springer ML, Wade J (1997) The effects of testicular tissue and prehatching inhibition of estrogen synthesis on the development of courtship and copulatory behavior in zebra finches. Horm Behav 32:46–59
Suthers RA (1997) Peripheral control and lateralization of birdsong. J Neurobiol 33:632–652
Sylvia VL, Boyan BD, Dean DD, Schwartz Z (2000) The membrane effects of 17β-estradiolon chondrocyte phenotypic expression are mediated by activation of protein kinase C through phospholipase C and G-proteins. J Steroid Biochem Mol Biol 73:211–224
Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z (2001) 17β-estradiol-BSA conjugatges and 17β-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem 81:413–429
Takahashi MM, Noumura T (1987) Sexually dimorphic and laterally asymmetric development of the embryonic duck syrinx: effect of estrogen on in vitro cell proliferation and chondrogenesis. Dev Biol 121:417–422
Toran-Allerand CD (2004) Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology 145:1069–1074
Turner RT, Riggs BL, Spelsberg TC (1994) Skeletal effects of estrogen. Endocr Rev 15:275–300
Veney SL, Wade J (2003) Sexually dimorphic neurocalcin expression in the developing zebra finch telencephalon. J Neurobiol 56:372–386
Veney SL, Wade J (2004) Steroid receptors in the adult zebra finch: a sex difference in androgen receptor mRNA, minimal expression of estrogen receptor α and aromatase. Gen Comp Endocrinol 136:192–199
Wade J (2001) Zebra finch sexual differentiation: the aromatization hypothesis revisited. Microsc Res Tech 54:354–363
Wade J, Arnold AP (2004) Sexual differentiation of the zebra finch song system. In: Zeigler HP, Marler P (eds) Behavioral neurobiology of birdsong, vol 1016. Ann N Y Acad Sci, New York, pp 540–559
Wade J, Buhlman L (2000) Lateralization and effects of adult androgen in a sexually dimorphic neuromuscular system controlling song in zebra finches. J Comp Neurol 426:154–164
Wade J, Schlinger BA, Hodges L, Arnold AP (1994) Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. Gen Comp Endocrinol 94:53–61
Wade J, Buhlman L, Swender D (2002) Post-hatching hormonal modulation of a sexually dimorphic neuromuscular system controlling song in zebra finches. Brain Res 929:191–201
Warner RW (1972) The anatomy of the syrinx in passerine birds. J Zool (Lond) 168:381–393
Acknowledgements
We thank Dr. Arthur Arnold (UCLA) for providing the templates for the zebra finch riboprobes, Dr. Fulton (Michigan State University, Department of Pathobiology and Diagnostic Investigation) for assistance with the identification of components of syrinx tissue, and Katie Grausam for help with histology. All procedures complied with the Principles of animal care, publication No. 86-23, revised 1985 of the National Institutes of Health and were approved by the Michigan State University All University Committee on Animal Use and Care. This work was supported by NSF DBI-0001973 to S.L.V. and R01-MH55488 and K02-MH065907 to J.W.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veney, S.L., Wade, J. Post-hatching syrinx development in the zebra finch: an analysis of androgen receptor, aromatase, estrogen receptor α and estrogen receptor β mRNAs. J Comp Physiol A 191, 97–104 (2005). https://doi.org/10.1007/s00359-004-0577-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-004-0577-5